This document discusses acid-base disorders and summarizes key points about acid-base balance, buffers, and the mechanisms that maintain homeostasis. It covers the respiratory, renal, and extracellular buffering systems and how they compensate for acid-base imbalances. Metabolic acidosis is discussed in depth, including causes like renal tubular acidosis and lactic acidosis. Treatment focuses on correcting the underlying disorder and using oral or intravenous sodium bicarbonate to gradually normalize pH.

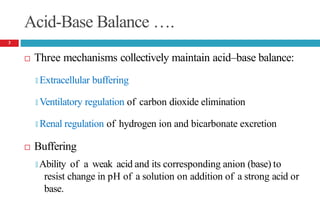
![Acid-Base Balance
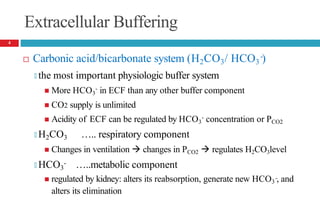
Acidity of body fluids is quantified by hydrogen ion
concentration
🞑 pH…… degree of acidity
🞑 Inverse relationship between [H+] and pH
Normal blood pH …..7.4
🞑 Used to analyze acid-base status
Hydrogen ion concentration in blood may not be indicative of
concentration in other body compartments
2](https://image.slidesharecdn.com/5-acid-base-2022-230511172011-6e47be52/85/5-Acid-Base-2022-pptx-2-320.jpg)
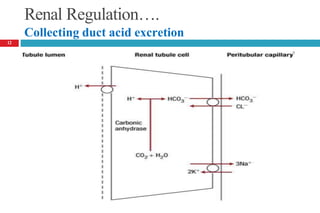
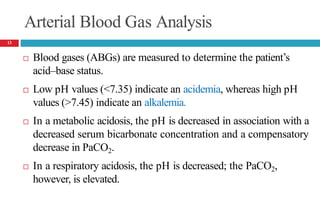
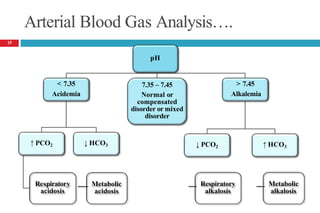
![Arterial Blood Gas Analysis….
In a metabolic alkalosis, the pH is elevated in association with
an increased bicarbonate concentration and a compensatory
increase in PaCO2.
In a respiratory alkalosis, the pH is also elevated; the PaCO2,
however, is decreased.
The normal values of each measurement;
🞑 pH = 7.4
🞑 PaCO2 = 40 mm Hg (5.3 kPa)
🞑 [HCO3
− ]= 24 mEq/L (24 mmol/L)
[PaCO : partial pressure of carbon dioxide]
14](https://image.slidesharecdn.com/5-acid-base-2022-230511172011-6e47be52/85/5-Acid-Base-2022-pptx-14-320.jpg)
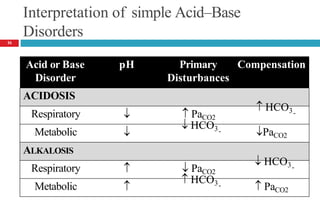

![Laboratory
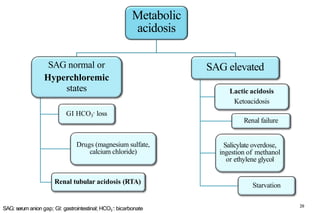
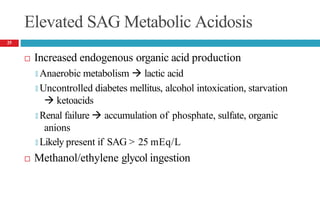
Serum anion gap (SAG)
🞑 SAG = [Na+] – [Cl-] – [HCO3
-]
🞑 SAG = [UAs] – [UCs]
🞑 Normal range 3 to 11 mEq/L (3 to 11 mmol/L)
🞑 High aniongap ---- accumulation of unmeasured anions in ECF.
[UAs: unmeasured anions, UCs: Unmeasured cations]
19](https://image.slidesharecdn.com/5-acid-base-2022-230511172011-6e47be52/85/5-Acid-Base-2022-pptx-19-320.jpg)

![Renal Tubular Acidosis (RTA)
Type I (distal)
🞑Decreased distal acidification
Urine pH during acidemia--- > 5.3
-
🞑Plasma [HCO3 ]---may be < 10 mEq/L
🞑Plasma [K+] ----usually reduced or normal
🞑Associated disease states
Tubule defect, Multiple myeloma, Systemic lupus erythematosus,
Sjögren’s syndrome, Sickle cell disease, Renal transplant rejection
following amphotericin B, Nephrocalcinosis
22](https://image.slidesharecdn.com/5-acid-base-2022-230511172011-6e47be52/85/5-Acid-Base-2022-pptx-22-320.jpg)
![Renal Tubular Acidosis….
Type II (proximal)
-
🞑 Decreased proximal HCO3 reabsorption
-
🞑 Plasma [HCO3 ] ---- usually 14–20 mEq/L
🞑 Urine pH during acidemia--- variable: > 5.3 or < 5.3
🞑 Plasma [K+] --- normal or reduced
🞑Associated disease states
Fanconi syndrome, Nephrotic syndrome, Paroxysmal nocturnal
hemoglobinuria, Carbonic anhydrase inhibitors, Impaired proximal
tubular glucose, phosphate, amino acid reabsorption
23](https://image.slidesharecdn.com/5-acid-base-2022-230511172011-6e47be52/85/5-Acid-Base-2022-pptx-23-320.jpg)
![Renal Tubular Acidosis….
Type IV (distal)
🞑Aldosterone deficiency or resistance
-
🞑 Plasma [HCO3 ] --- usually >15 mEq/L
🞑 Urine pH during acidemia---- <5.3
🞑 Plasma [K+] ----- Elevated
🞑Associated disease states
Primary mineralocorticoid deficiency (Addison disease)
Hyporeninemic hypoaldosteronism (diabetic nephropathy, mild
renal impairment)
Impaired tubular potassium secretion and urinary obstruction
24](https://image.slidesharecdn.com/5-acid-base-2022-230511172011-6e47be52/85/5-Acid-Base-2022-pptx-24-320.jpg)
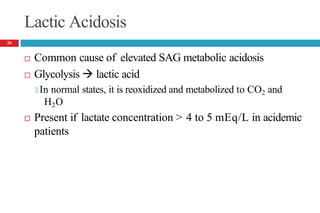
![Lactic Acidosis Causes
Decrease in tissue oxygenation [tissue hypoxia] (type A)
🞑 Shock, Severe anemia, CHF, CO poisoning
Deranged oxidative metabolism (type B)
🞑 Medications
Catecholamines, metformin, NRTIs
Overdose (iron, isoniazid, salicylates, theophylline)
Propofol infusion syndrome, Propylene glycol toxicity, Na nitroprusside
🞑 Methanol, ethanol, ethylene glycol
🞑 Diabetes mellitus, Malignancy
🞑 Disorders associated with inborn errors of metabolism
27](https://image.slidesharecdn.com/5-acid-base-2022-230511172011-6e47be52/85/5-Acid-Base-2022-pptx-27-320.jpg)
![Oral Alkali Replacement
- - -
LD = VDHCO3 x BW x (desired [HCO3 ] – current [HCO3 ])
-
E.g., If 60 kg and HCO3 is 15 mEq/L
🞑 LD = (0.5 L/kg x 60 kg) x (24 mEq/L – 15 mEq/L)
= 30 L x 9 mEq/L
= 270 mEq/L
Give doseoverseveral daysto avoidvolume overloadfrom the
accompanyingNa load.
31
LD: loading dose; VD: volume of distribution; BW: body weight](https://image.slidesharecdn.com/5-acid-base-2022-230511172011-6e47be52/85/5-Acid-Base-2022-pptx-31-320.jpg)

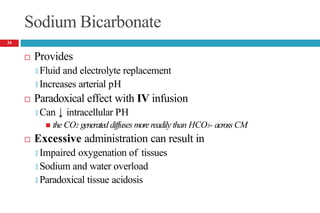
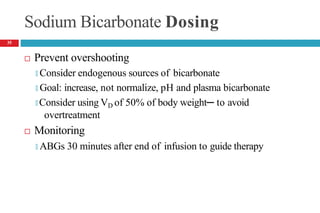

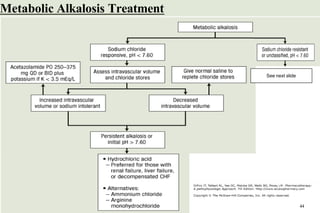
![METABOLIC ALKALOSIS
Pathophysiology
It is an acid–base disorder that presents as alkalemia (↑d
arterial pH) with an increase in plasma bicarbonate.
Evaluation of patients with metabolic alkalosis must consider
two separate issues:
🞑 the initial process that generates the metabolic alkalosis
🞑 alterations in renal function that maintain the alkalemic state
Initial interpretation of labs
🞑 PaCO2 (mmHg) should increase by 0.4 to 0.6 times the rise in
plasma [HCO3
-]
37](https://image.slidesharecdn.com/5-acid-base-2022-230511172011-6e47be52/85/5-Acid-Base-2022-pptx-37-320.jpg)

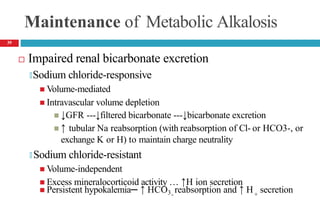


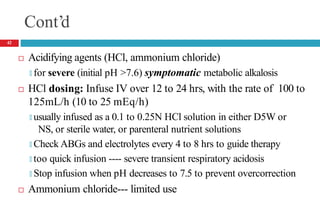


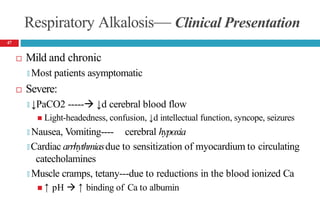
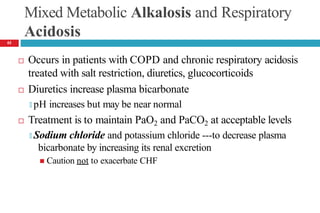
![Respiratory Alkalosis— Clinical Presentation
Laboratory Tests
🞑 Serum chloride concentration usually slightly ↑d
🞑 ↓d serum ionized Ca , potassium, phosphorus concentrations
Acute alkalosis: Plasma [HCO -] should decrease by 0.2 times
3
the decrease in PaCO2 but usually not to <18 mEq/L
Chronic alkalosis: Plasma [HCO -] should fall by 0.35 times
3
the decrease in PaCO2 but usually not to <14 mEq/L
48](https://image.slidesharecdn.com/5-acid-base-2022-230511172011-6e47be52/85/5-Acid-Base-2022-pptx-48-320.jpg)
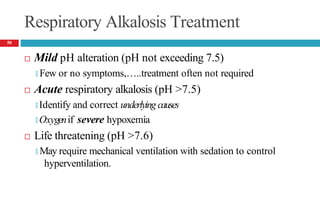

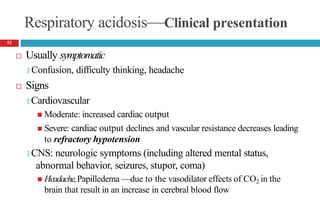
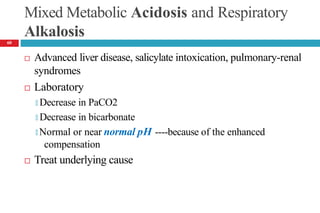
![Cont’d
Laboratory tests
🞑 Moderate increase in serum potassium
🞑 Moderate to severe hypercapnia
PaCO2 = 50 to 55 mm Hg (moderate), PaCO2 >80mm Hg (severe)
🞑 Hypoxia often present (PaO2 <70 mm Hg)
🞑 Acute acidosis: plasma [HCO3-] should increase by 0.1 times the
increase in PaCO2
🞑 Chronic acidosis: plasma [HCO3-] should increase by 0.35 times the
increase in PaCO2
53](https://image.slidesharecdn.com/5-acid-base-2022-230511172011-6e47be52/85/5-Acid-Base-2022-pptx-53-320.jpg)
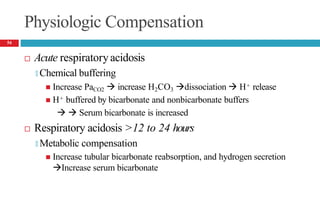
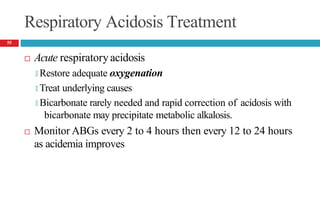
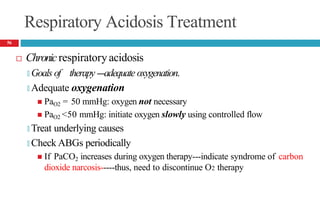

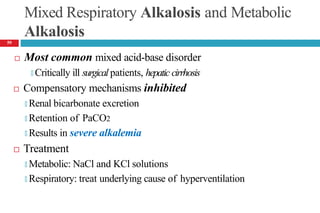
![Mixed Respiratory Acidosis and Metabolic Acidosis
Develop in cardiopulmonary arrest, chronic lung disease, shock,
and in metabolic acidosis pts who develop respiratory failure
Compensatory mechanisms inhibited
🞑 Respiratory decrease in PaCO2
🞑 Buffering and renal mechanisms that increase bicarbonate
🞑 Thus, the pH decreases markedly.
Treatment
🞑 Oxygenation [to improve hypercarbia and hypoxia]
🞑 Alkali to reverse the metabolic acidosis
58](https://image.slidesharecdn.com/5-acid-base-2022-230511172011-6e47be52/85/5-Acid-Base-2022-pptx-58-320.jpg)