This document discusses various spectroscopy techniques including absorption spectroscopy, infrared spectroscopy, ultraviolet spectroscopy, and mass spectroscopy. It provides details on:
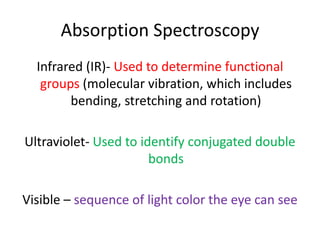
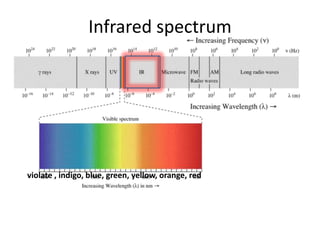
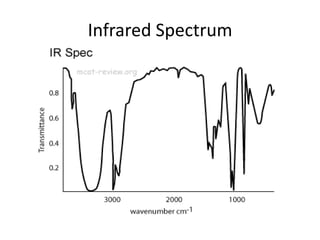
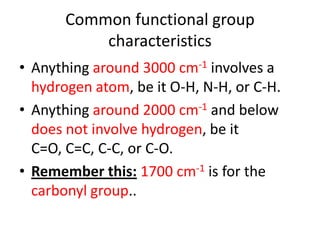
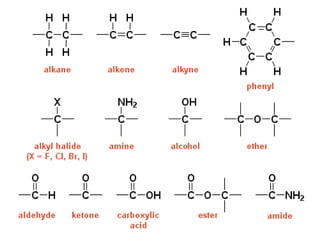
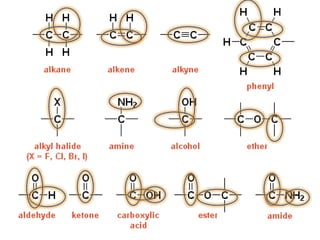
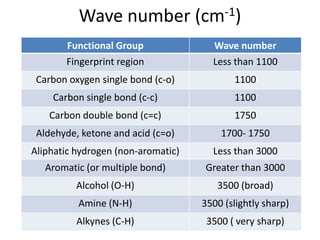
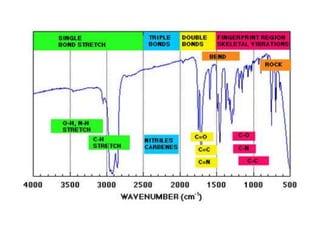
- Infrared spectroscopy is used to determine functional groups through molecular vibrations and rotations that can be stretching, bending, or compressing.
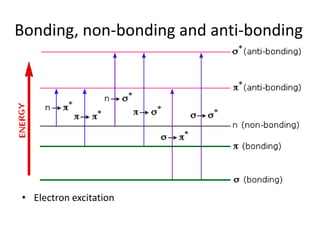
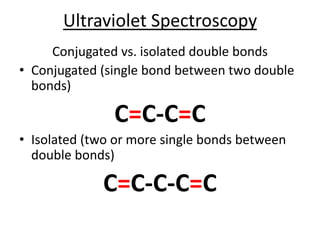
- Ultraviolet spectroscopy identifies conjugated double bonds through electron transitions.
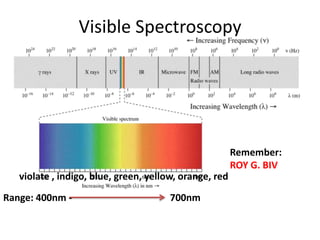
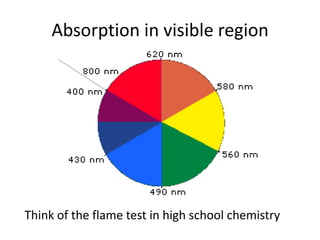
- Visible spectroscopy determines color absorption in the visible light range.
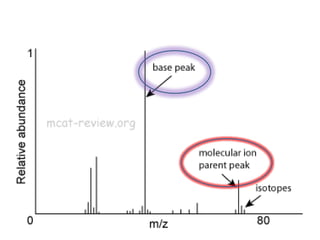
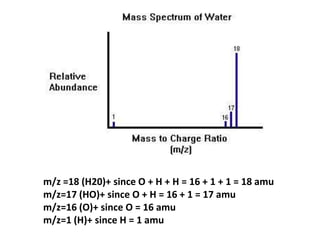
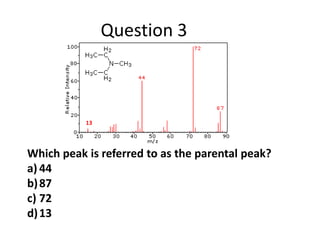
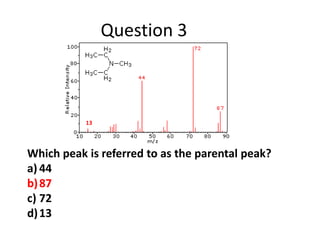
- Mass spectroscopy identifies molecular formulas and masses by bombarding samples and analyzing mass-to-charge ratios of fragment ions.