This document provides information on interpreting arterial blood gases (ABGs), including:
1) How to identify acidosis or alkalosis based on pH levels.
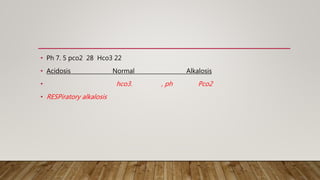
2) The Rome method for determining if a pH change is due to respiratory or metabolic causes based on pCO2 and HCO3 levels.
3) Examples of respiratory acidosis, respiratory alkalosis, and metabolic alkalosis and how they may present on ABGs.
4) How the body can partially or fully compensate for acid-base imbalances through changes in HCO3 or pCO2.
5) Formulas like Winter's formula and the Anion Gap that can help evaluate compensation and identify causes of metabolic acidosis