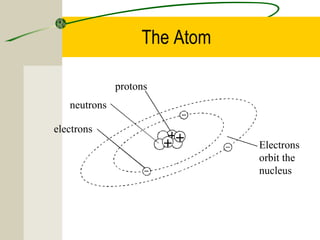
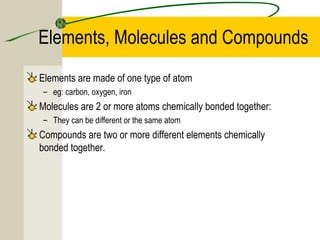
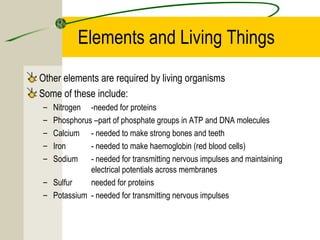
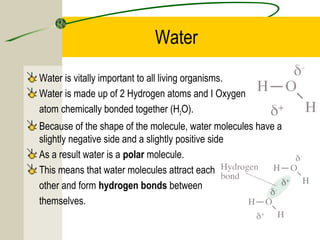
The most common chemical elements in living things are carbon, hydrogen, oxygen, and nitrogen. Other important elements include sulfur, calcium, phosphorus, iron, and sodium, each playing roles like being part of proteins or bones. Water has a polarity that allows it to form hydrogen bonds between molecules, giving it properties useful for living things like being a solvent, coolant, and transport medium for metabolic reactions.