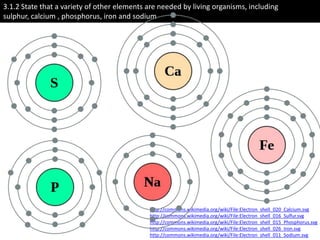
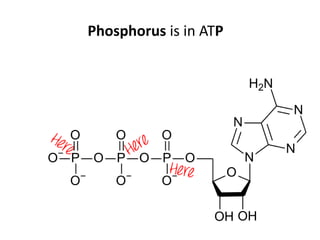
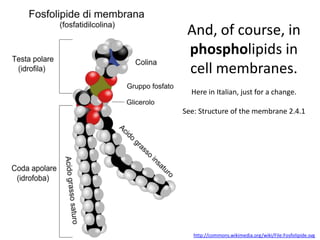
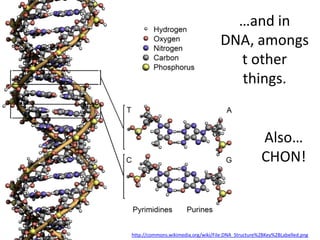
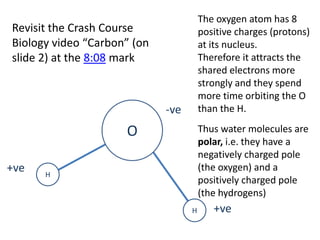
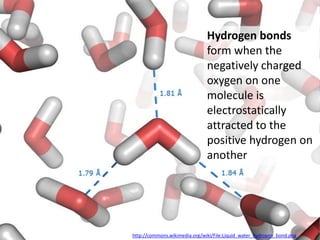
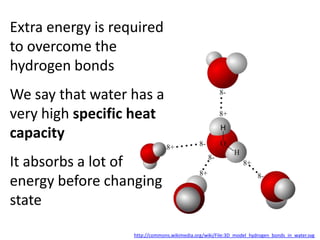
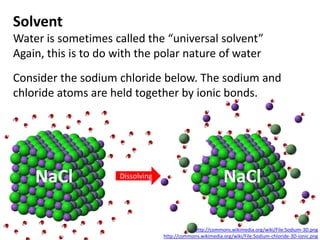
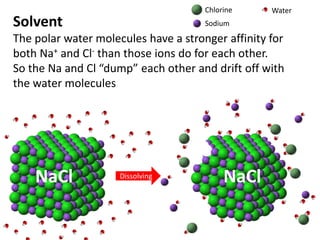
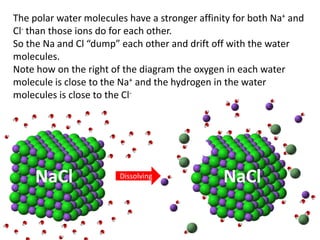
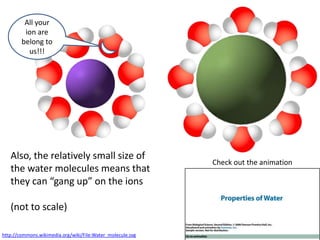
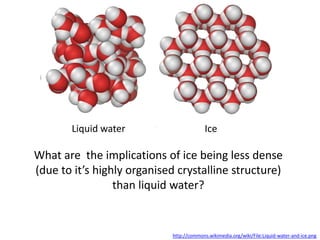
The document discusses the properties of key elements found in living things and water. It states that carbon, hydrogen, oxygen and nitrogen are the most common elements, while sulfur, calcium, phosphorus, iron and sodium are also needed. It then provides examples of the role of each element, including sulfur in amino acids, iron in hemoglobin, calcium in bones, sodium for nerve impulses, phosphorus in ATP and DNA. The document also discusses the polarity and hydrogen bonding of water molecules and how this leads to water's thermal, cohesive and solvent properties, which allow it to act as a coolant, medium for reactions and transport in organisms.