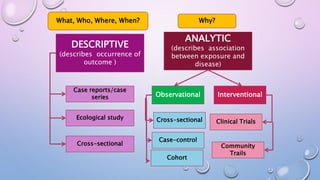
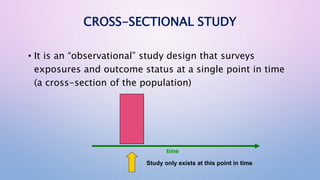
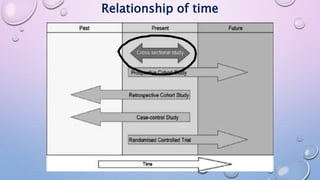
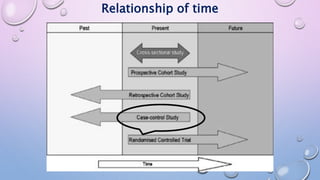
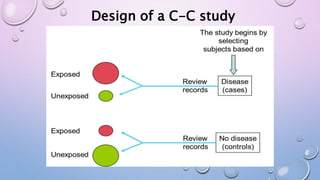
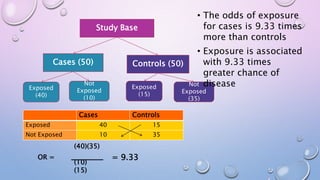
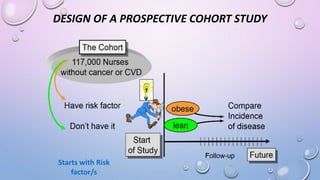
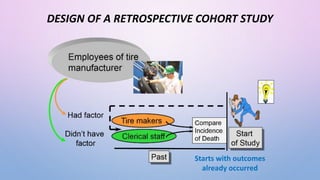
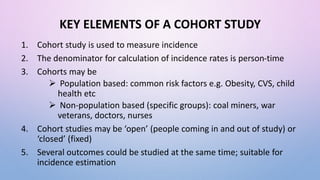
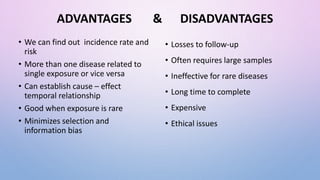
The document discusses various observational study designs in community dentistry, focusing on cross-sectional, case-control, and cohort studies. It outlines the characteristics, methodologies, advantages, and disadvantages of each study type, emphasizing their applications in understanding disease prevalence, risk factors, and causal relationships. Additionally, it provides insights into implementing these studies, including sampling, data collection, and ethical considerations.