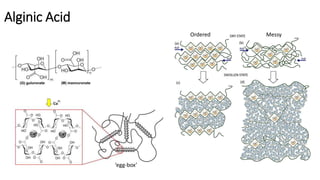
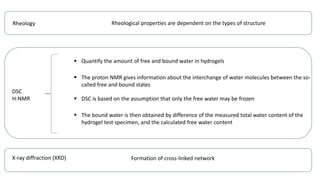
This document discusses hydrogels, which are 3D polymer networks that can absorb large amounts of water while maintaining their shape. It provides a brief history of hydrogels and classifications based on generation. Stimuli-responsive or "smart" hydrogels that change properties in response to environmental stimuli are highlighted. Characterization techniques and applications of hydrogels in biomedical areas like drug delivery, cell encapsulation, and tissue engineering scaffolds are summarized.




























![References
Cheung et al., J Biotechnol Biomater 2015, 5:2
J Appl Biotechnol Rep. 2018 Sep;5(3):81-91
Trends in Biotechnology June 2015, Vol.33,No. 6
Journal of Advanced Research (2015) 6, 105-121
Material Science and Engineering C 57 (2015) 414-433
Polymers 2017, 9, 137; doi:10.3390/polym9040137
Talebian, Set al., (2019). Self-Healing Hydrogels: The Next Paradigm Shift in Tissue Engineering? Advanced Science, 6(16),
[1801664]
Analyst, 2003, 128, 325–331](https://image.slidesharecdn.com/hydrogels-201007151437/85/Hydrogels-31-320.jpg)