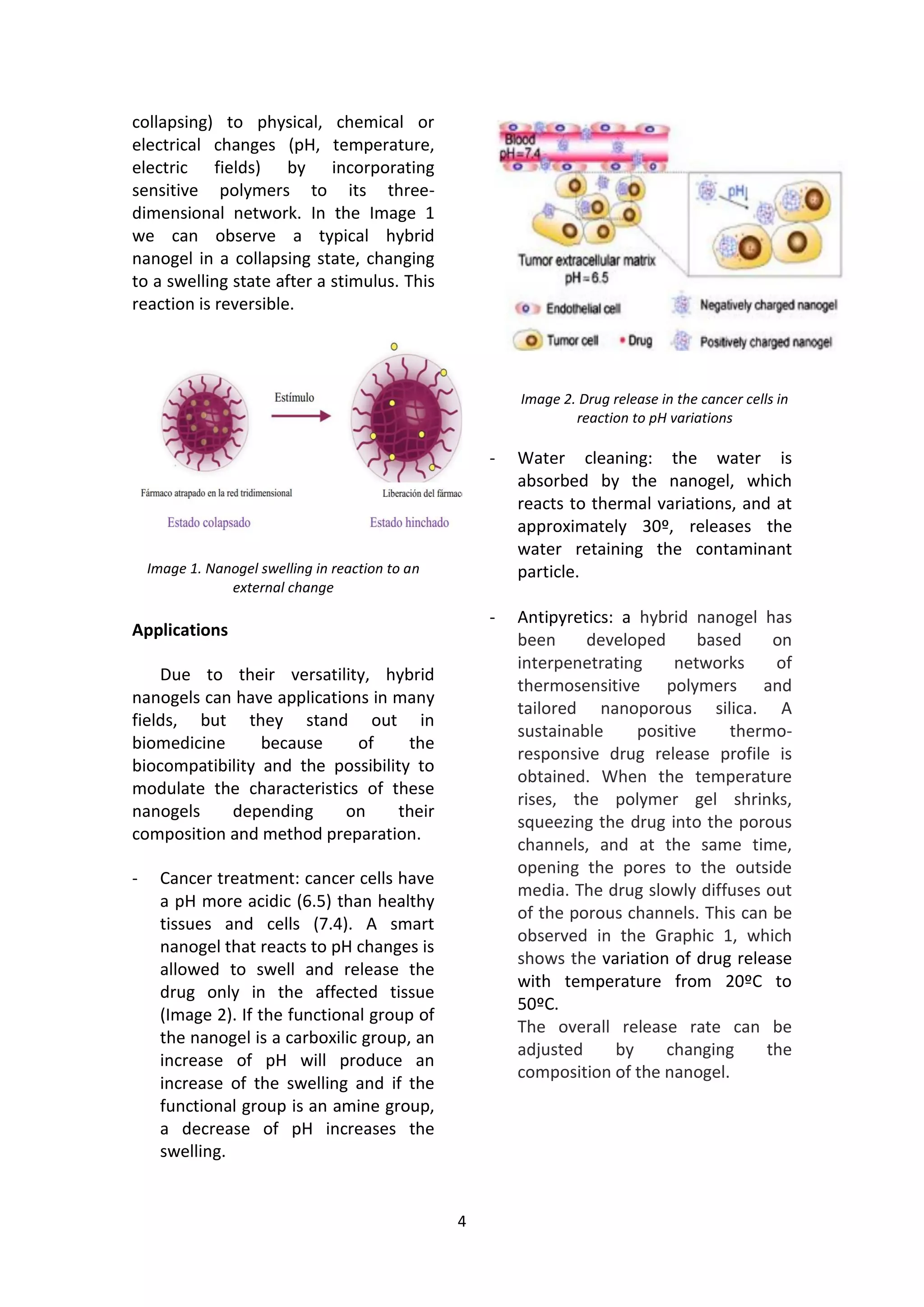
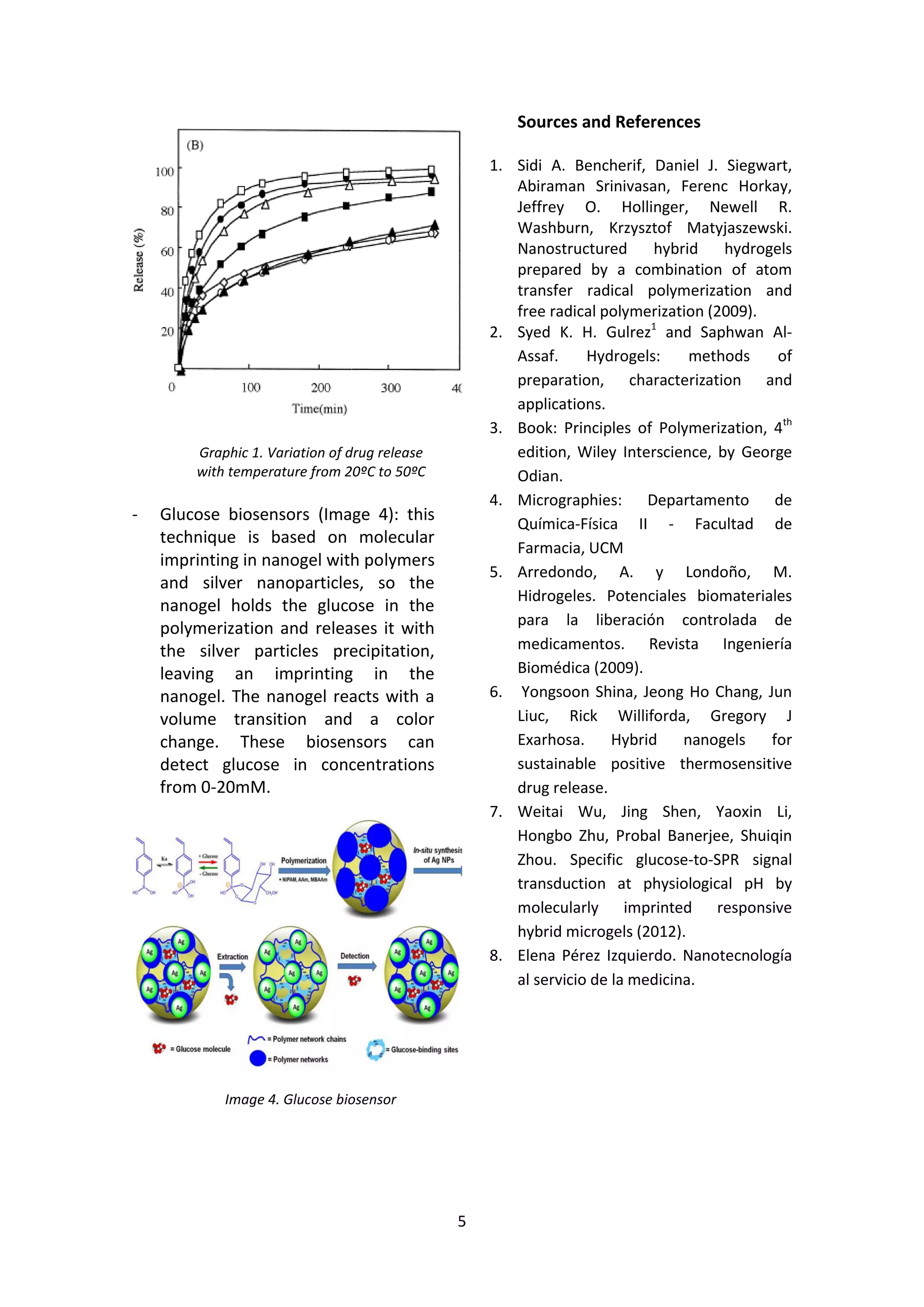
Nanogels are nanosized hydrogels that can be synthesized through various polymerization techniques. They have potential applications in drug delivery, tissue engineering, and bionanotechnology due to their 3D structure, mechanical properties, and biocompatibility. Hybrid nanogels are smart materials that can swell or collapse in response to physical or chemical stimuli like pH, temperature, or electric fields. This makes them useful for applications like cancer treatment by releasing drugs only in acidic tumor environments, water purification by absorbing contaminants, and glucose biosensing through a volume change signal.