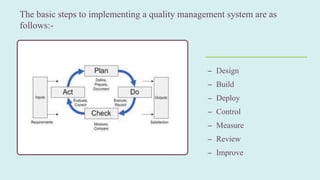
The document, presented by Vijay Kumar, discusses the Quality Management System (QMS) in the pharmaceutical industry, emphasizing its role in improving product quality and minimizing recall risks. It outlines essential elements such as quality policies, risk management, objectives, and the importance of quality manuals, procedures, and work instructions tailored to organizational needs. The document emphasizes that a structured approach is crucial for effective implementation of a QMS, incorporating elements like responsibilities, document control, and continual improvement.