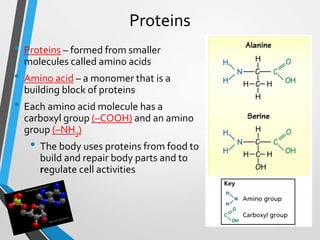
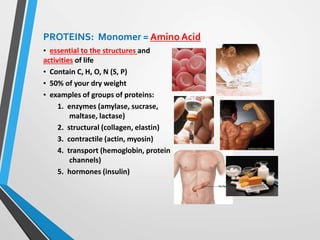
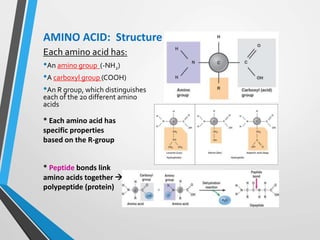
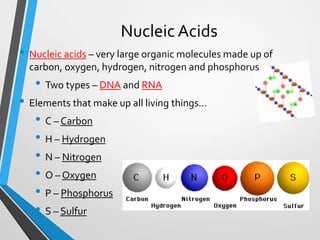
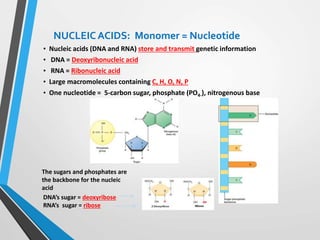
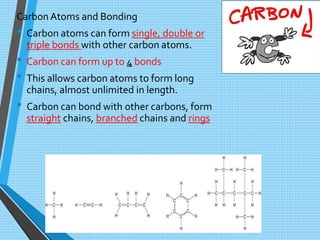
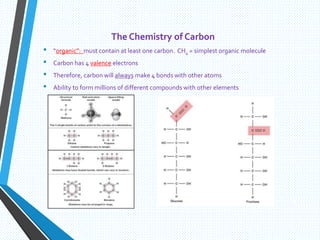
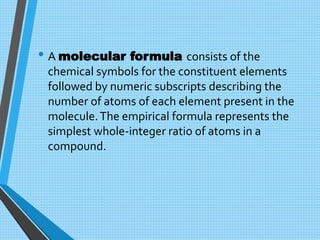
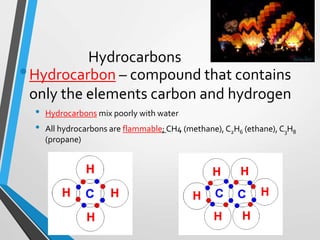
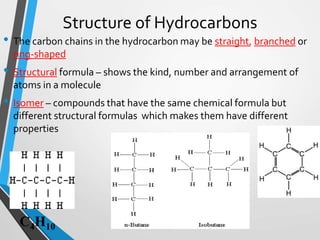
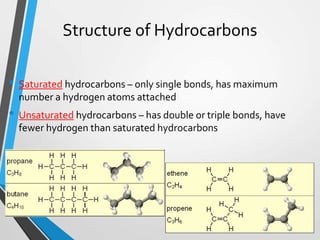
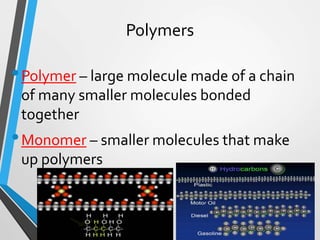
The document discusses the importance of organic compounds and their various classes, including carbohydrates, proteins, lipids, and nucleic acids, which are essential for all living things. It outlines the structures, functions, and examples of these macromolecules, as well as the properties of carbon that enable a wide variety of organic compounds. It also covers the chemistry of hydrocarbons, isomers, and the role of vitamins, minerals, and water in biological processes.