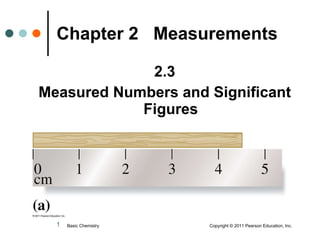
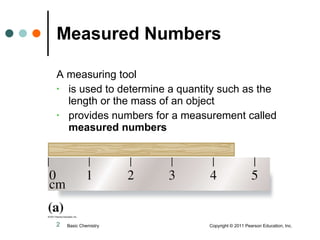
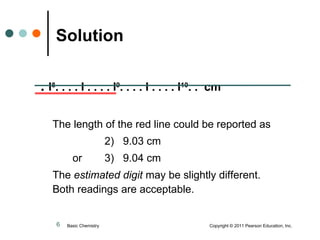
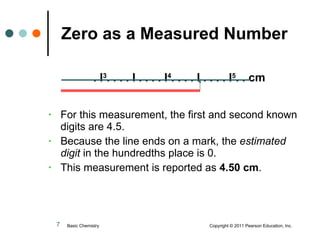
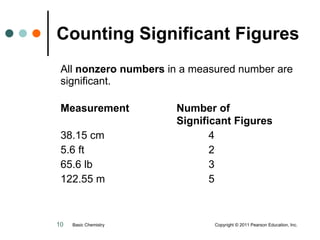
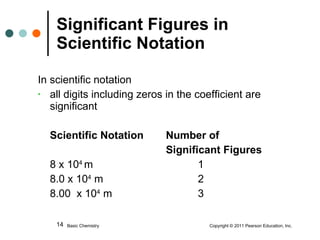
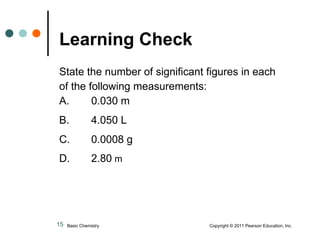
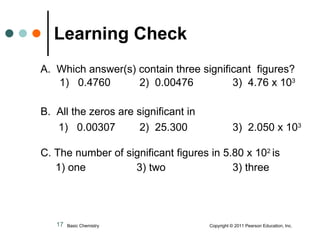
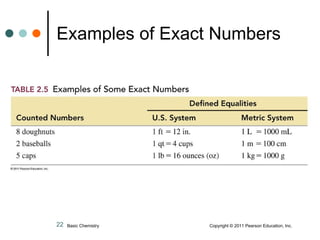
This document discusses measured numbers and significant figures in measurements. It defines measured numbers as numbers provided from a measurement using a measuring tool. It explains that measured numbers have known digits that are certain, and may have estimated digits that are uncertain. All digits in a measured number, including estimated digits, are considered significant figures. The number of significant figures reported depends on the precision of the measuring tool used. It provides examples of how to determine the number of significant figures in various measurements.