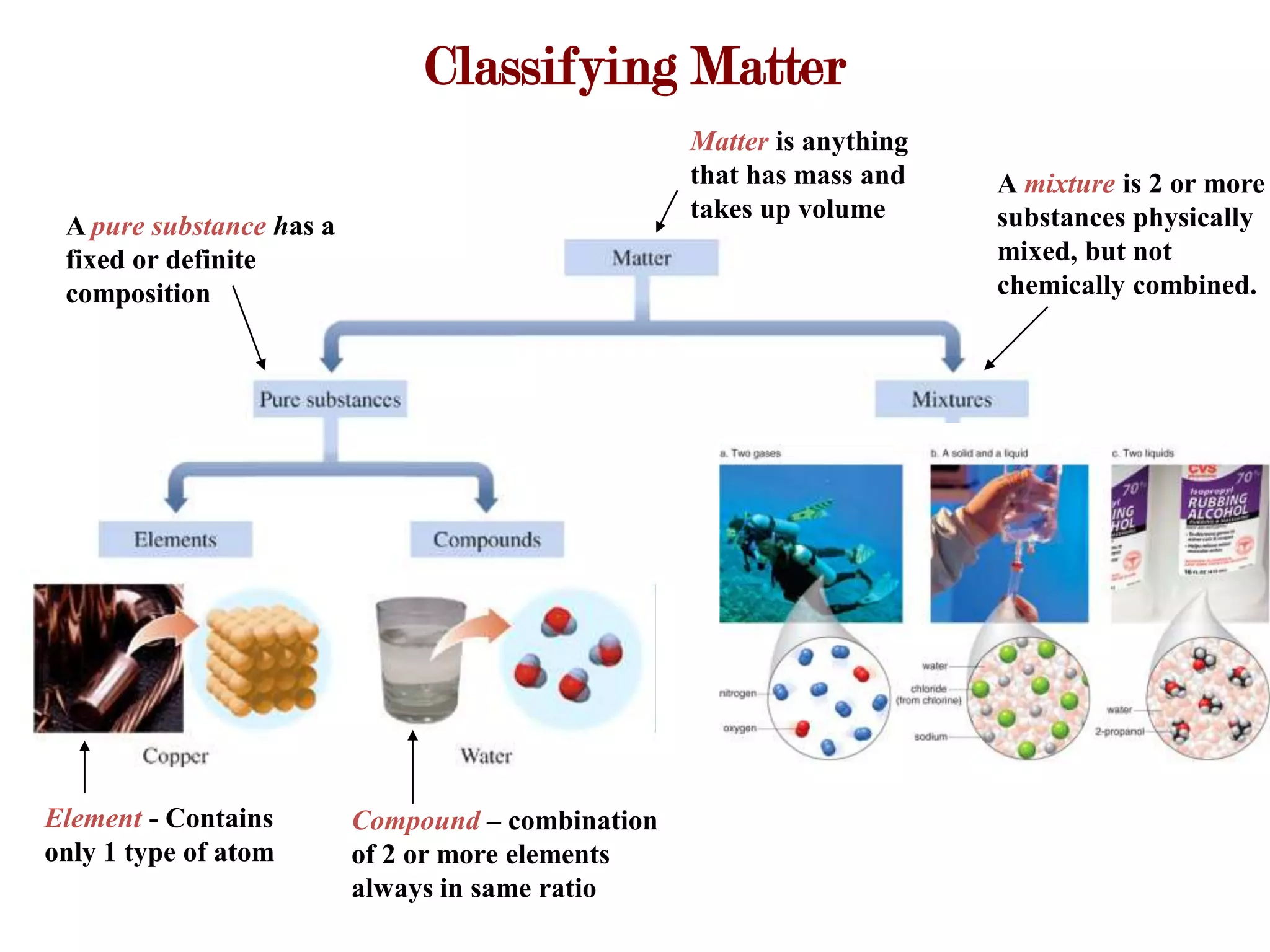
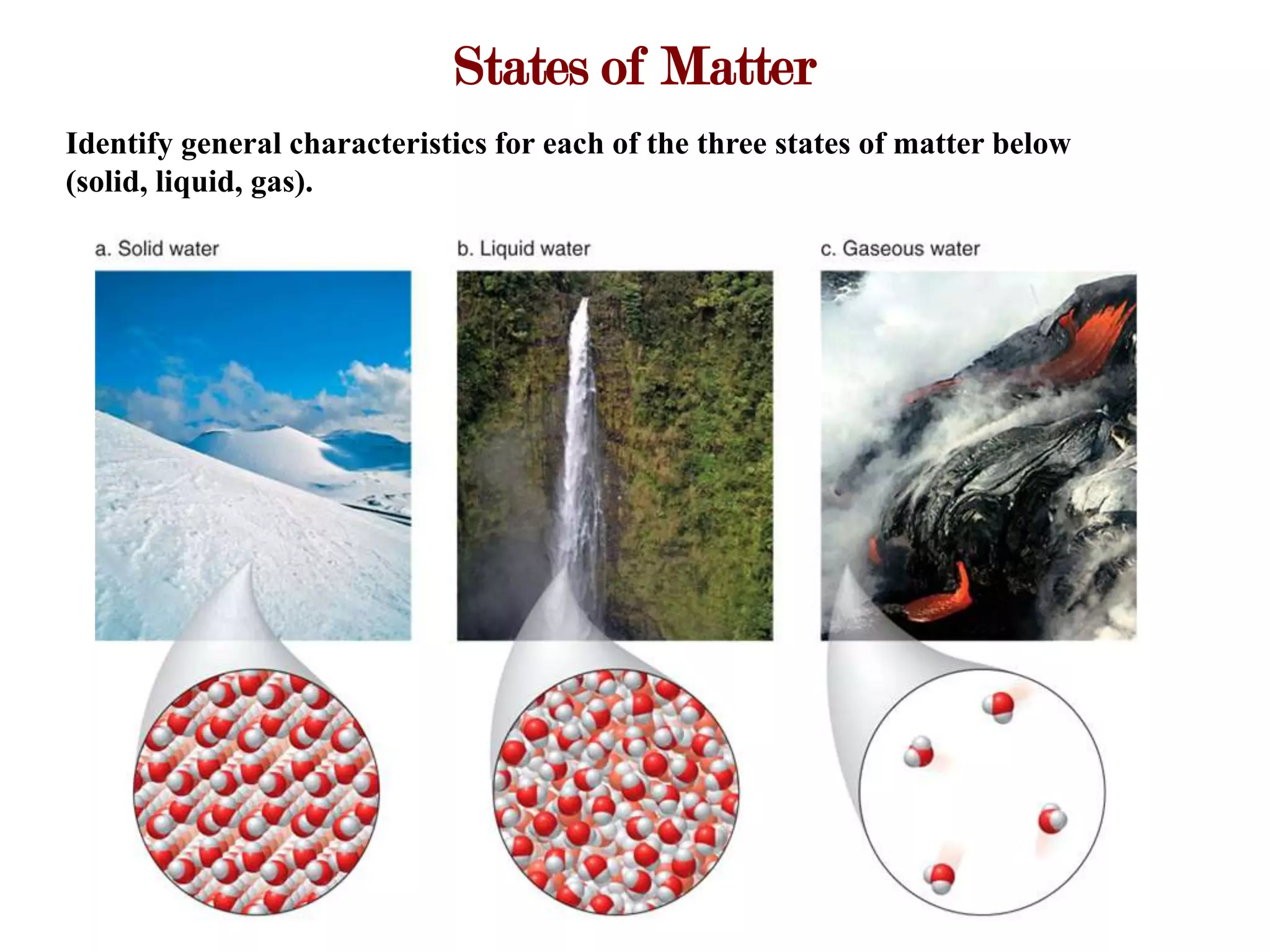
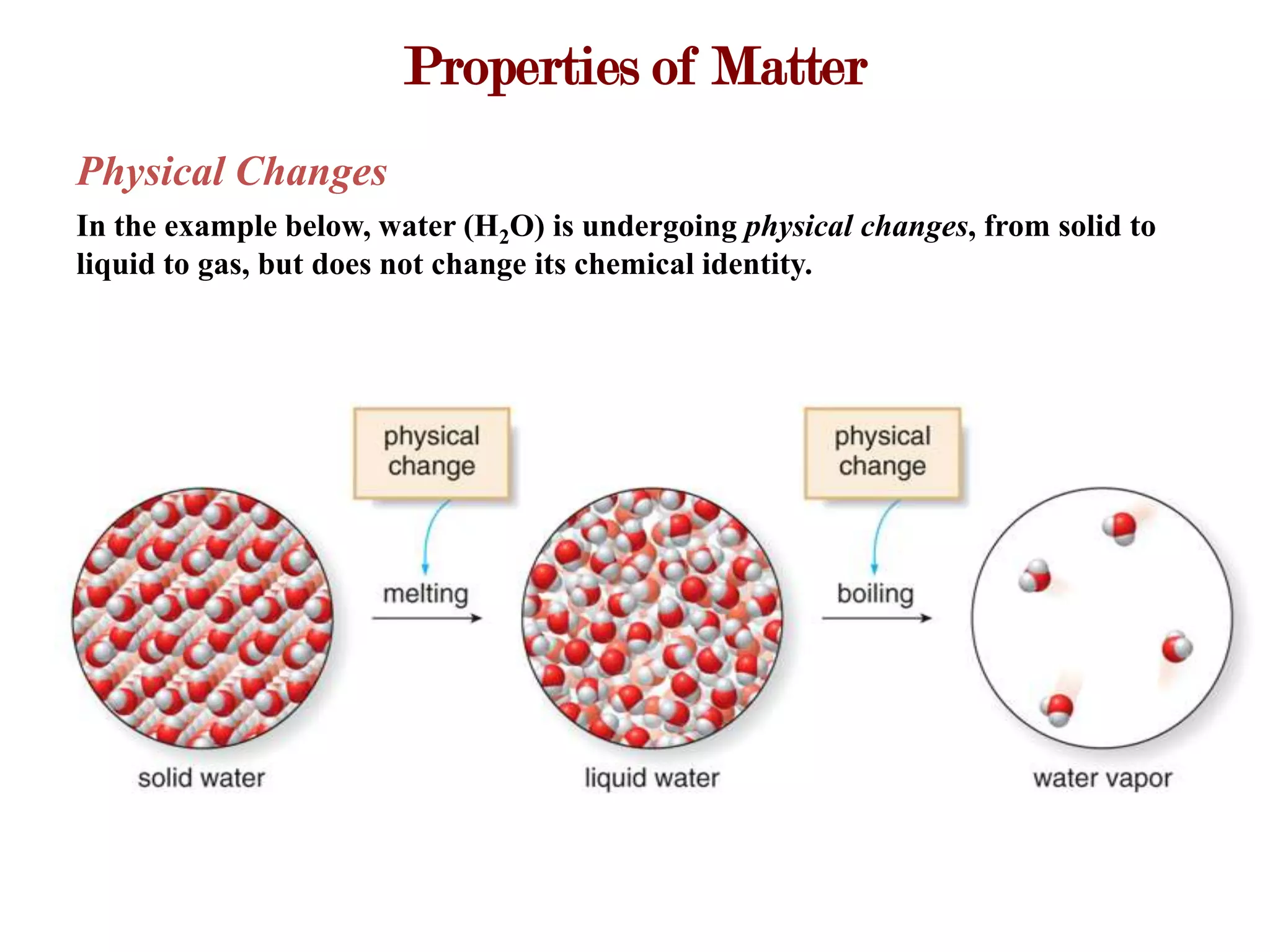
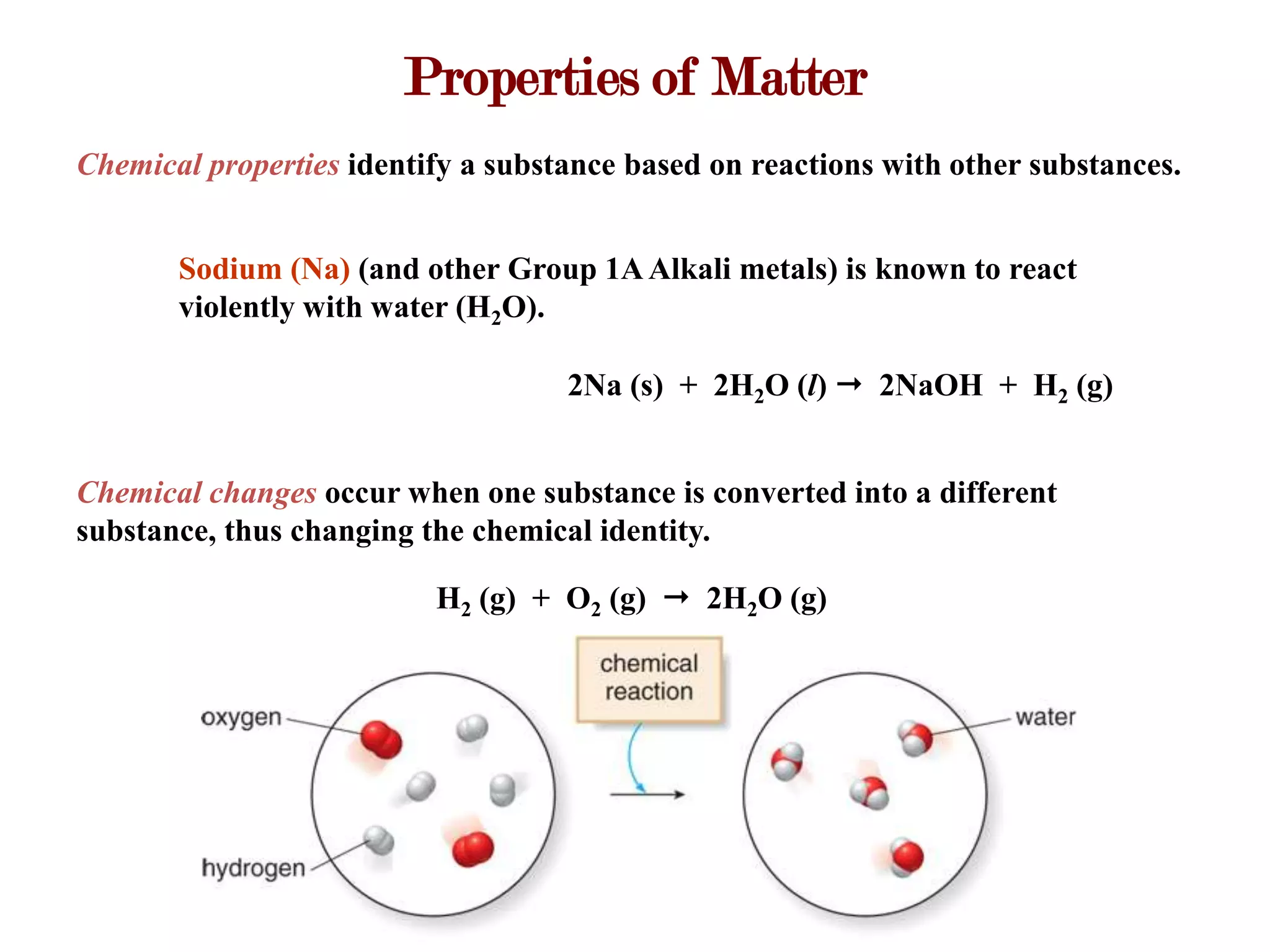
This document provides an overview of key concepts in classifying and measuring matter, including:
- Pure substances can be elements or compounds, while mixtures contain two or more substances physically mixed.
- The three common states of matter (solid, liquid, gas) have general characteristic properties.
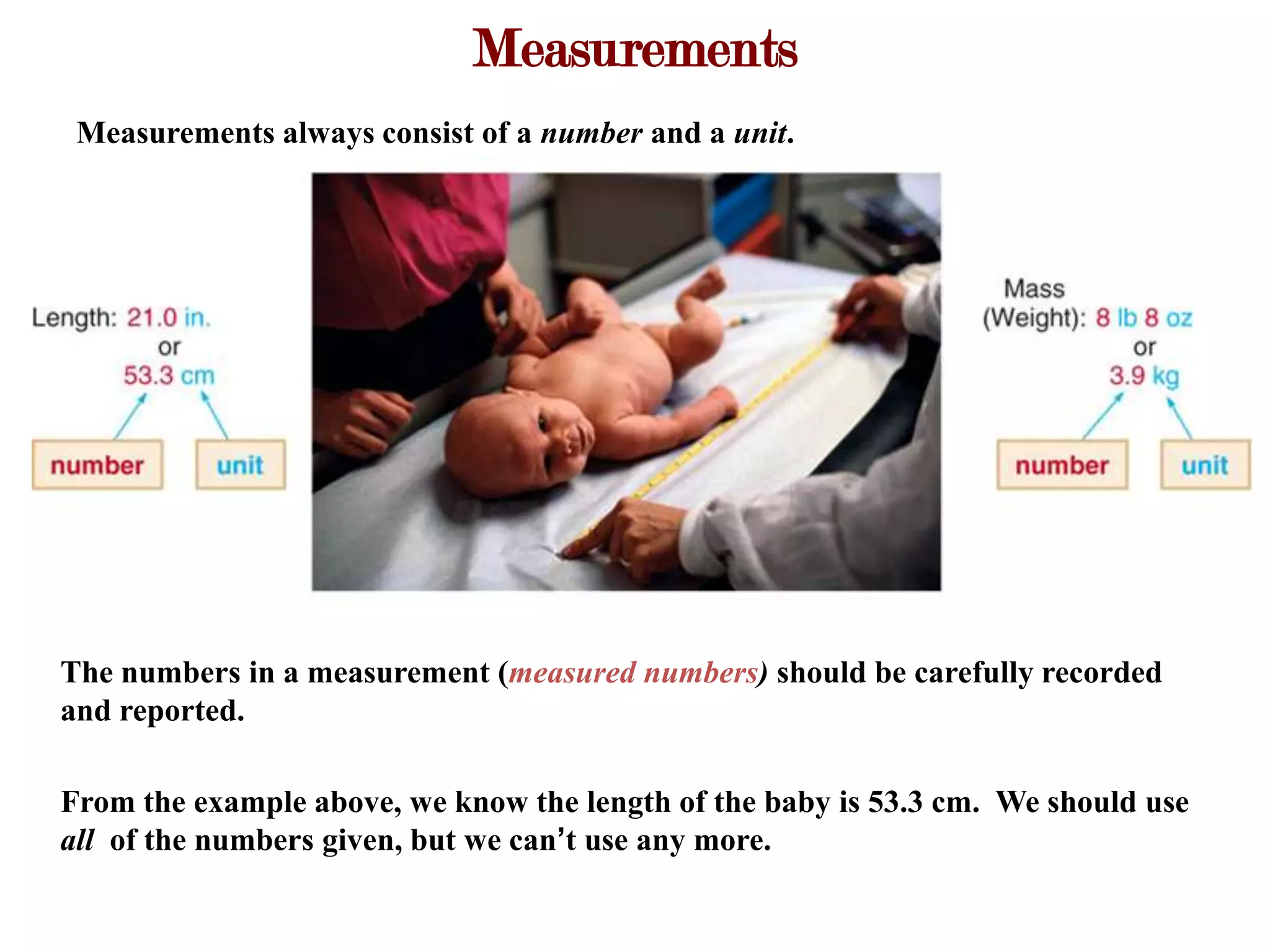
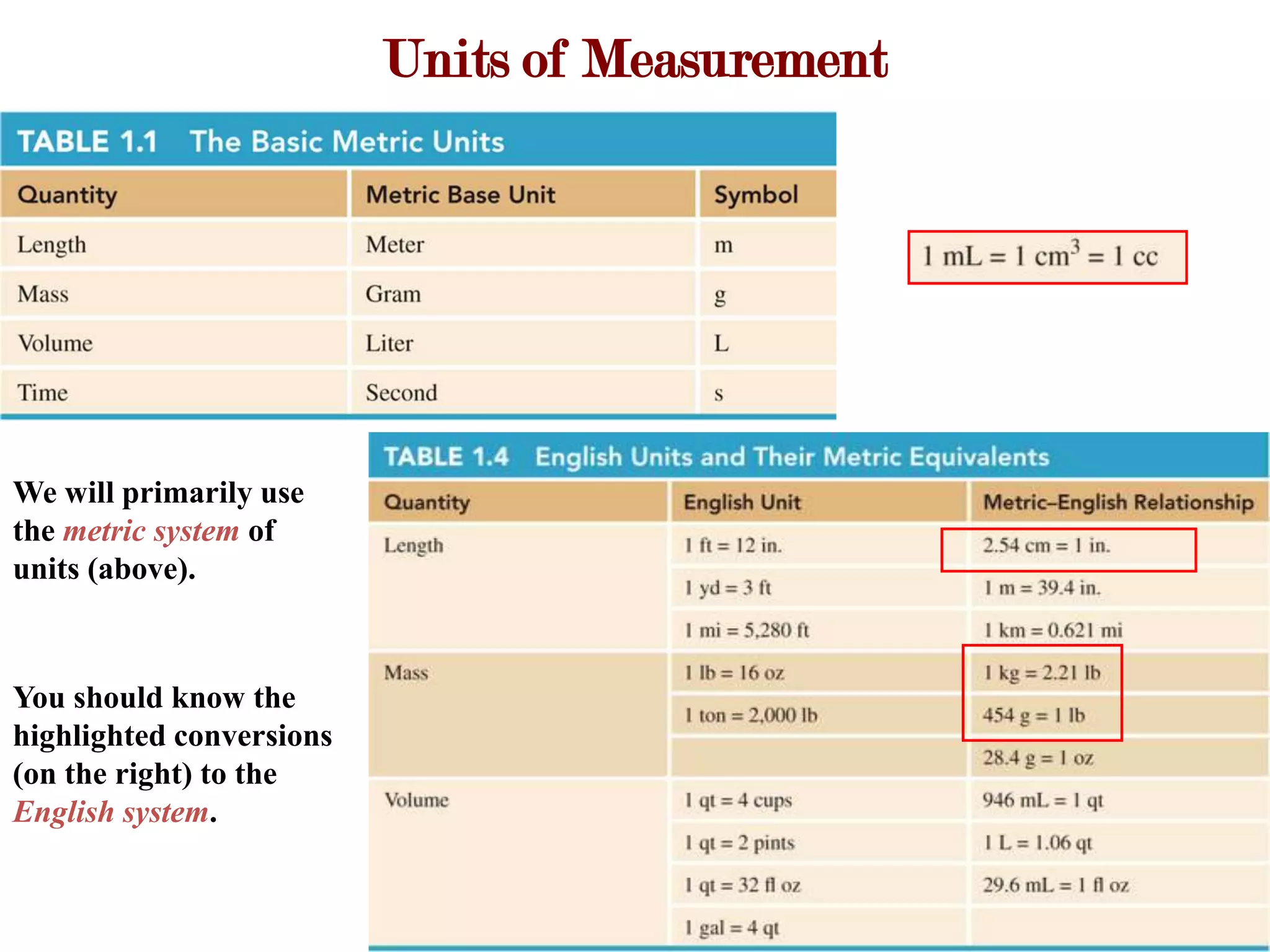
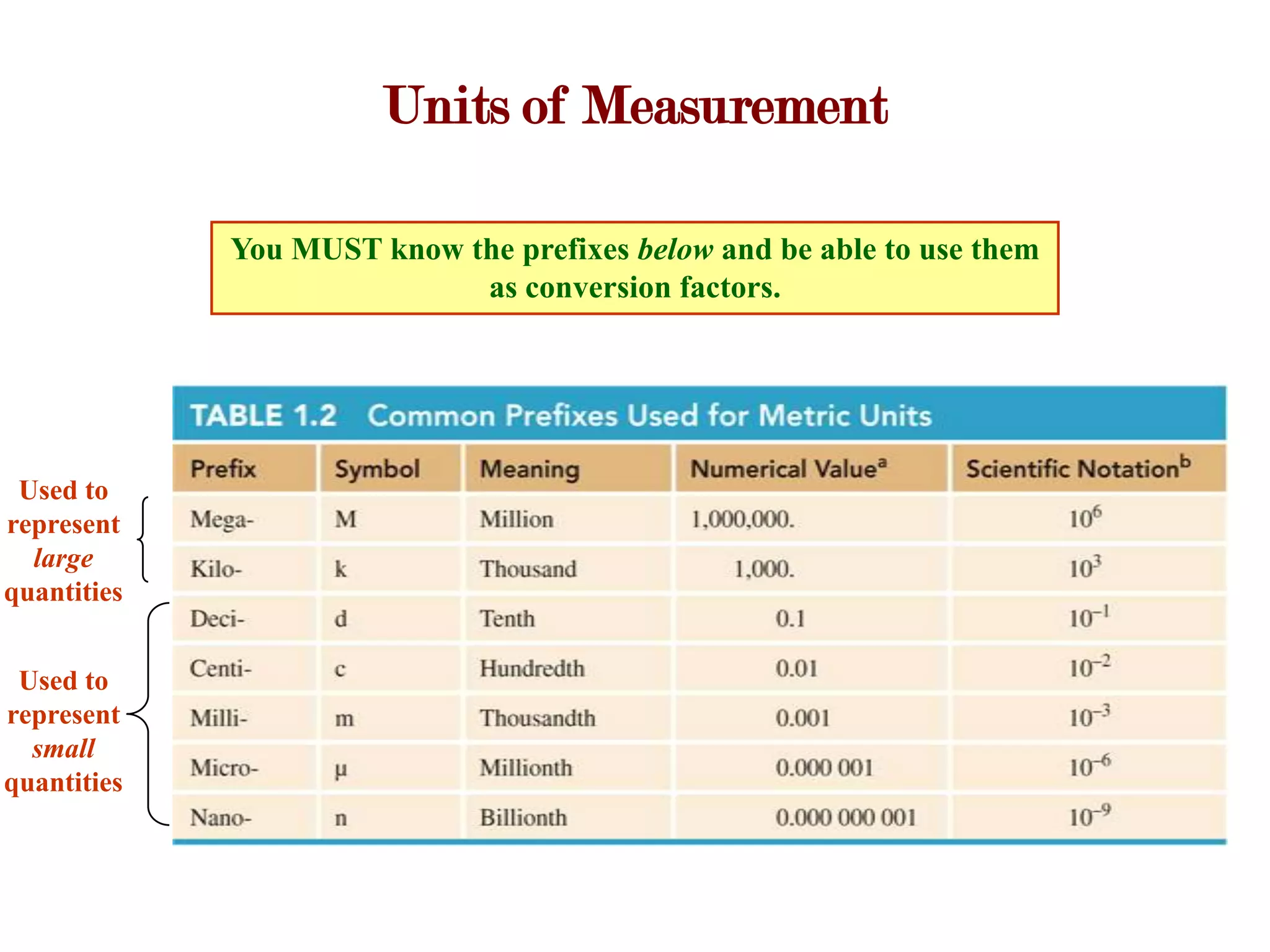
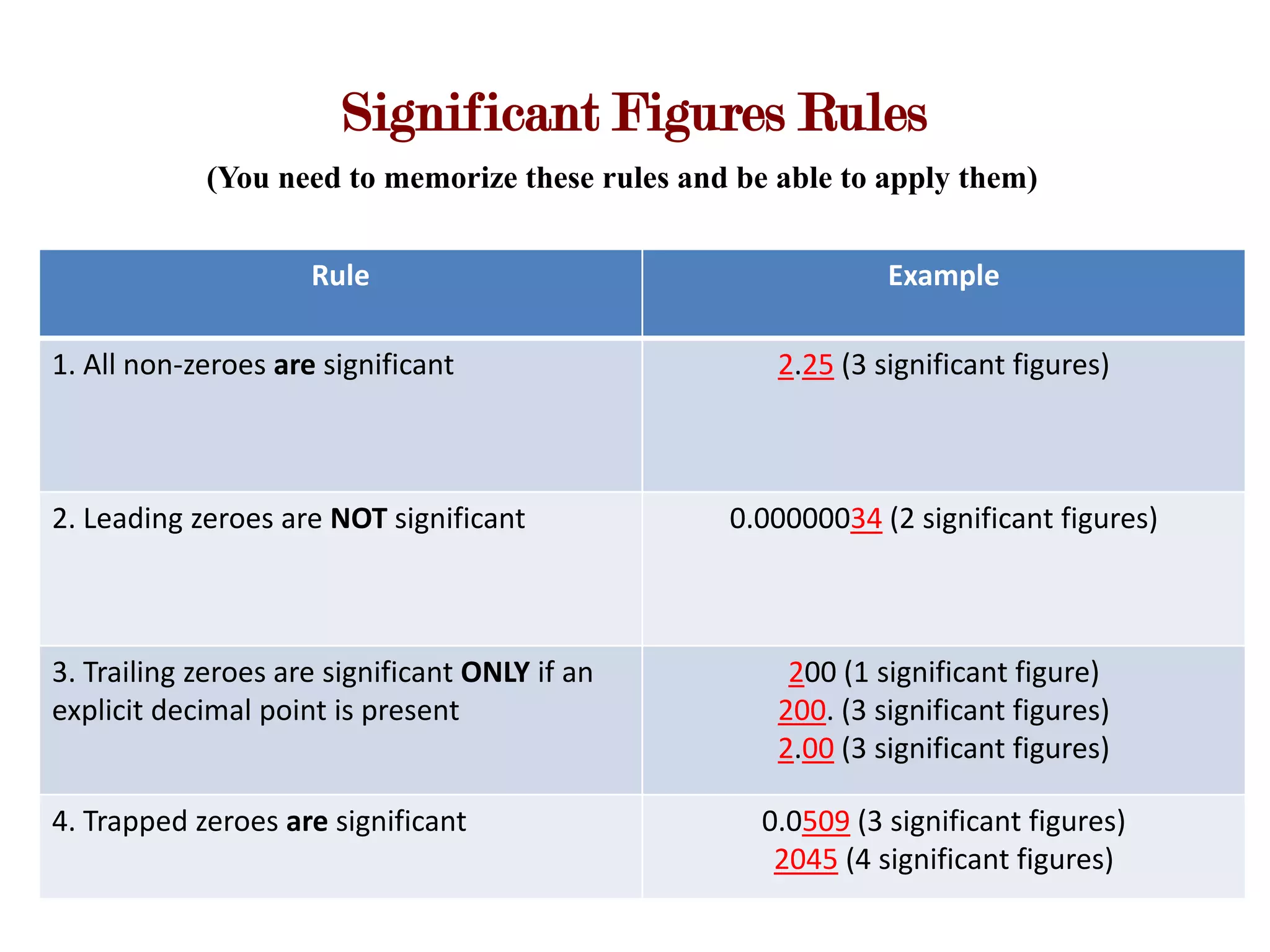
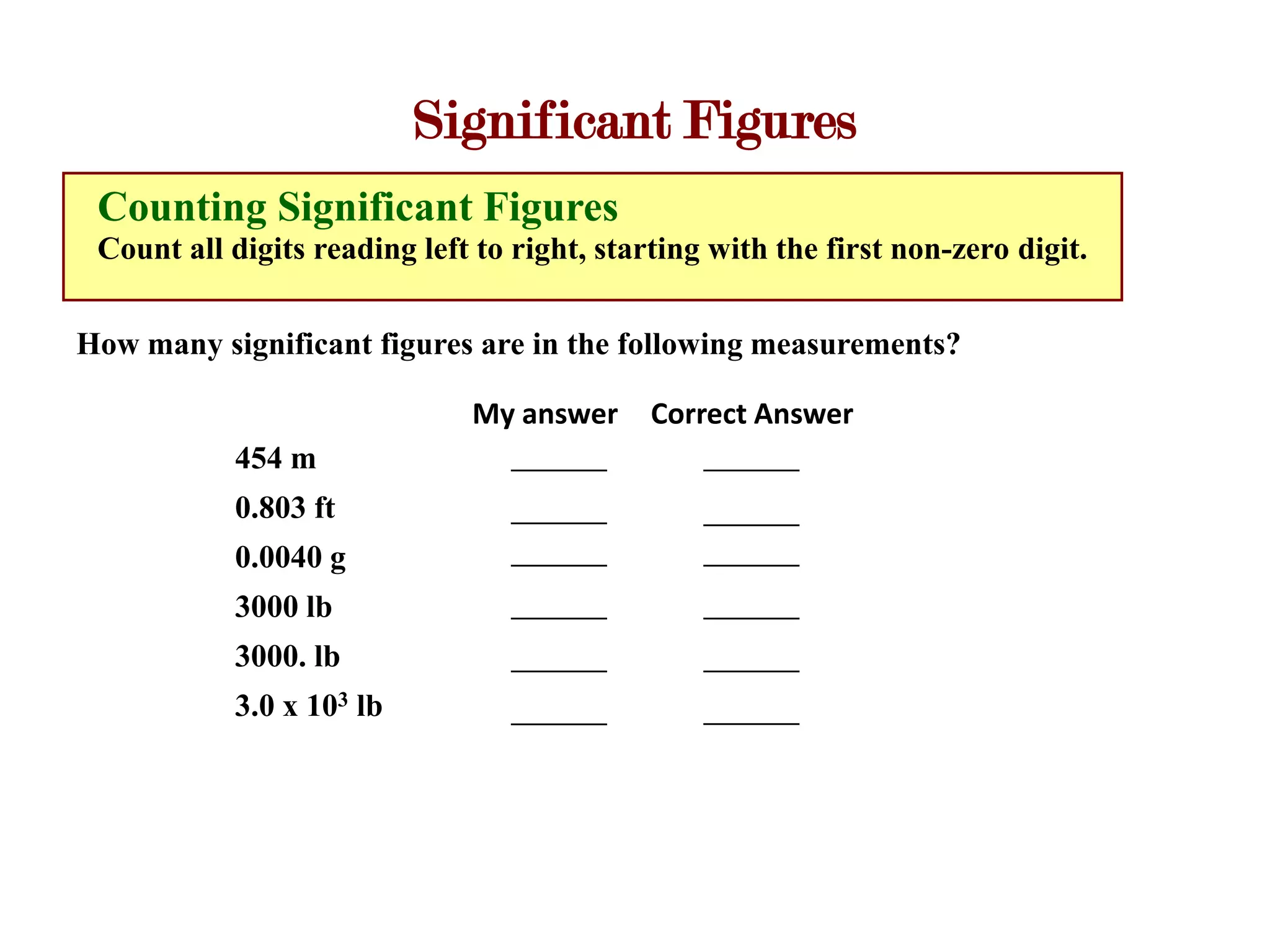
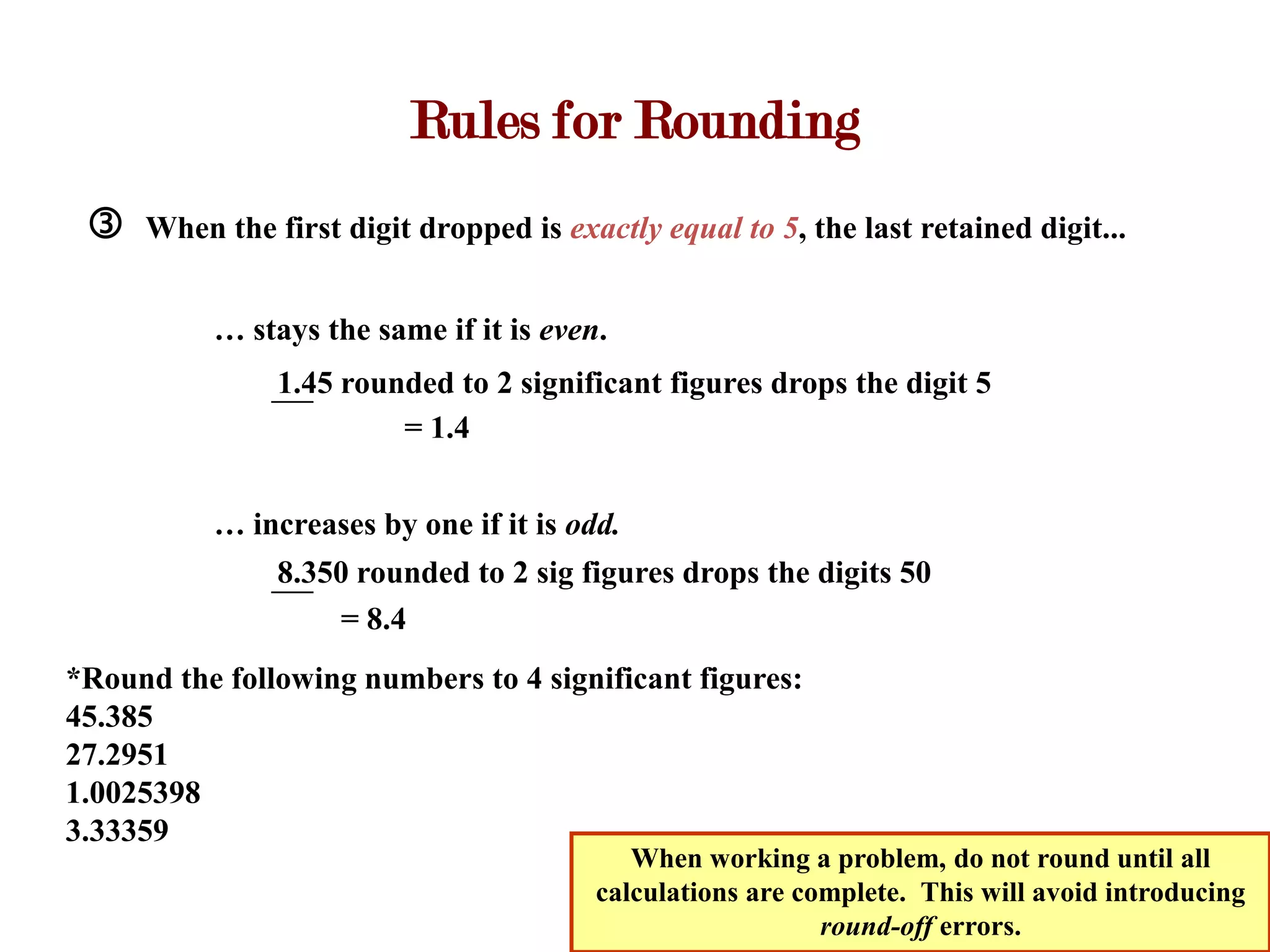
- Measurements involve a number and unit, and must be reported with the correct number of significant figures based on measurement precision.
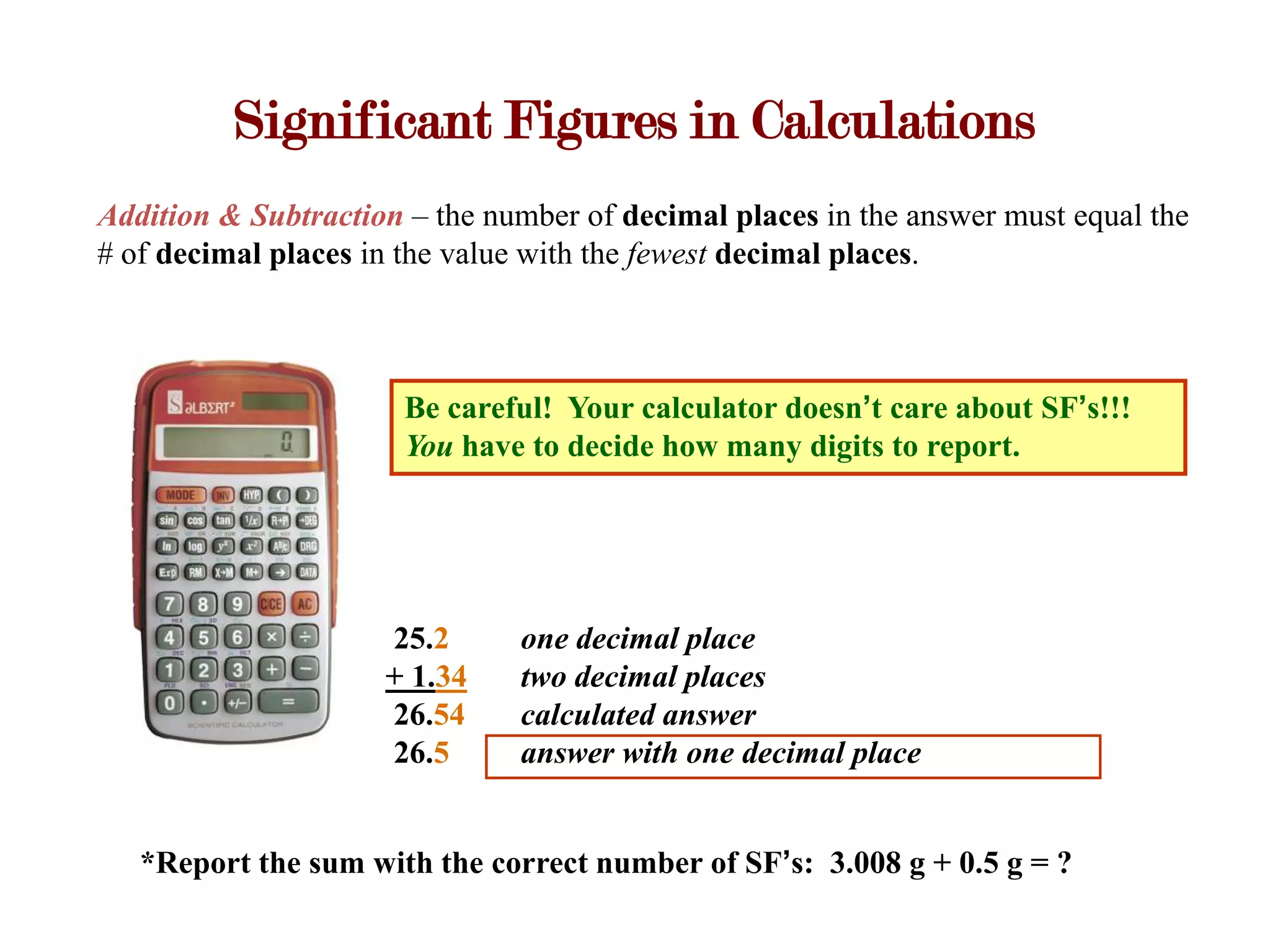
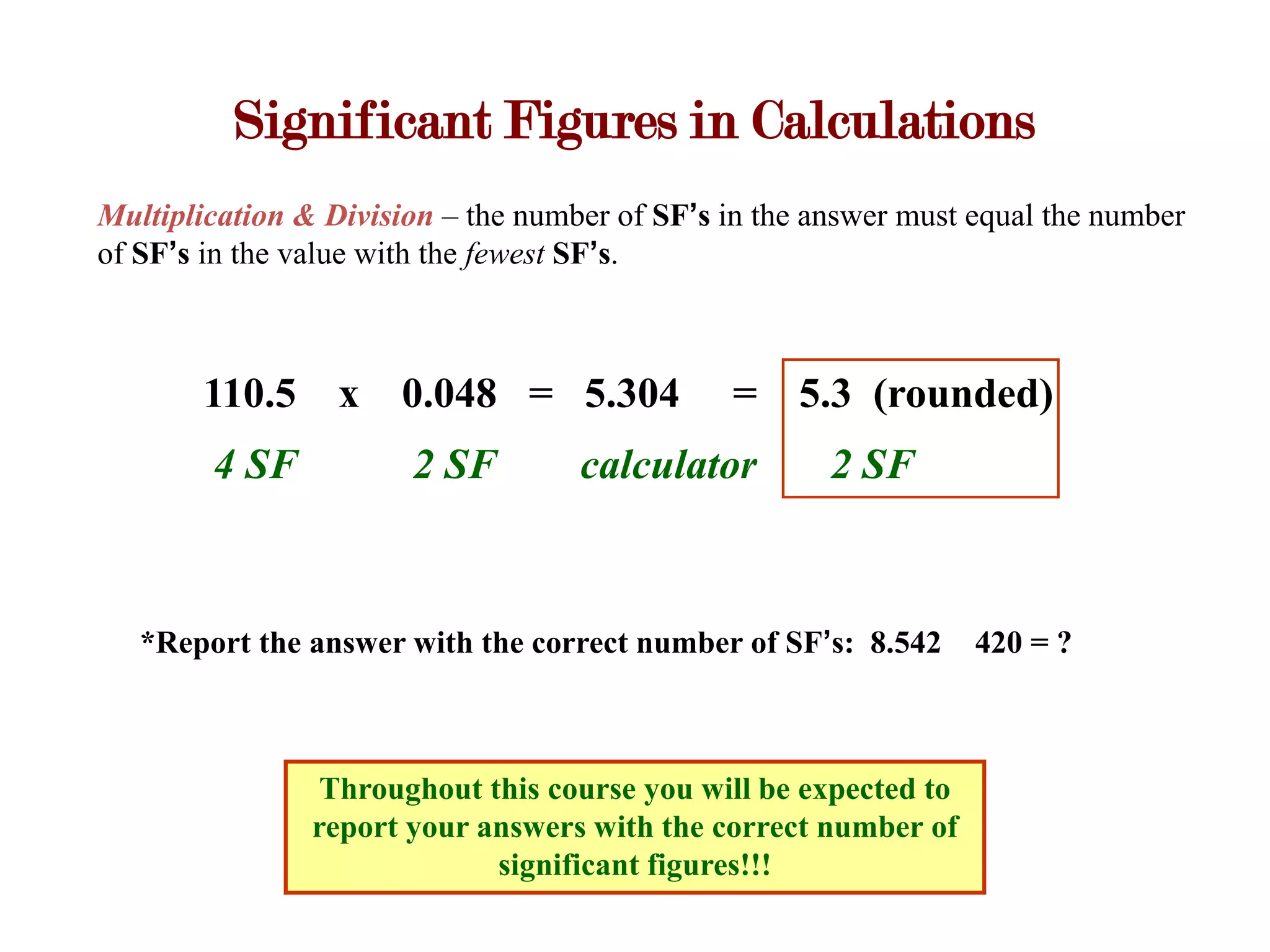
- Calculations with addition, subtraction, multiplication, and division require applying significant figure rules to determine the correct number of figures in the final answer.