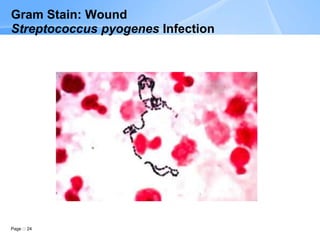
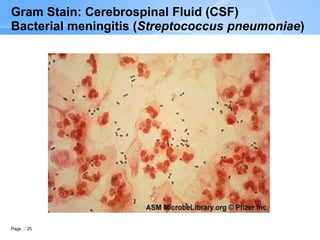
The document describes the Gram stain procedure and interpretation. It discusses how Gram staining classifies bacteria based on differences in cell wall structure and composition that determine whether they appear gram-positive or gram-negative. The Gram stain procedure and interpretation are explained in detail over multiple pages, including slide preparation, staining steps, microscopic evaluation, and reporting of results. Examples of various bacteria visualized by Gram stain from different specimen types are also shown.