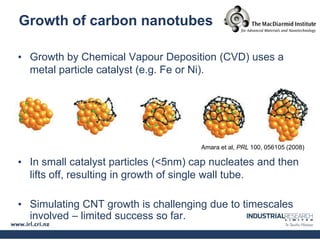
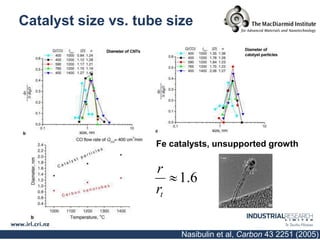
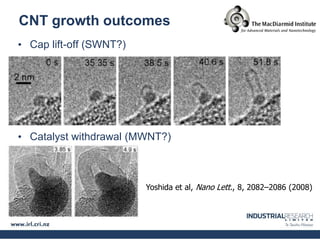
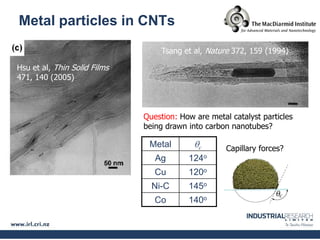
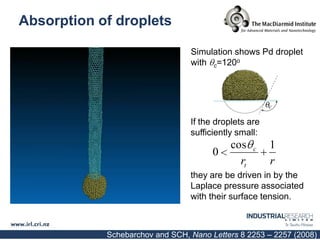
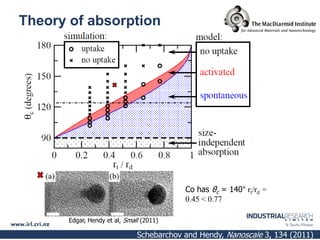
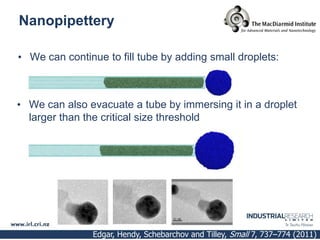
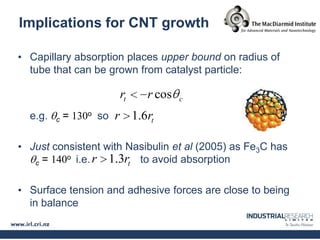
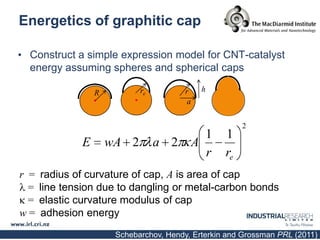
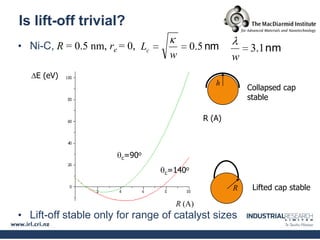
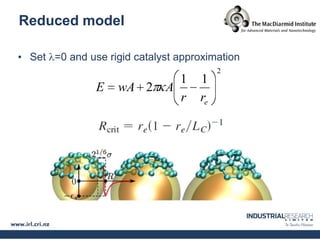
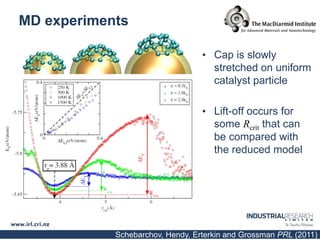
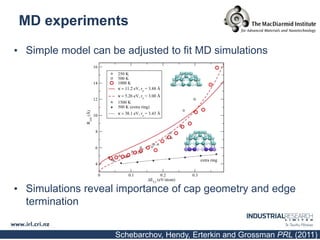
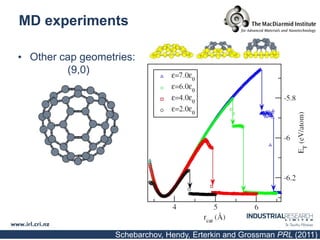
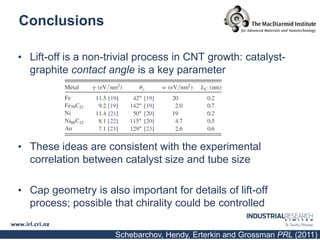
Carbon nanotubes are grown using chemical vapor deposition with a metal catalyst particle. Simulations show the carbon nanotube cap controls the chirality and diameter of the grown nanotube. For a nanotube to lift off and grow, the radius of the catalyst particle must be within a specific range determined by the particle's contact angle. The energetics of cap formation and lift off can be modeled and show good agreement with molecular dynamics simulations. Understanding how the cap detaches from the particle may allow controlling the chirality of the grown carbon nanotube.