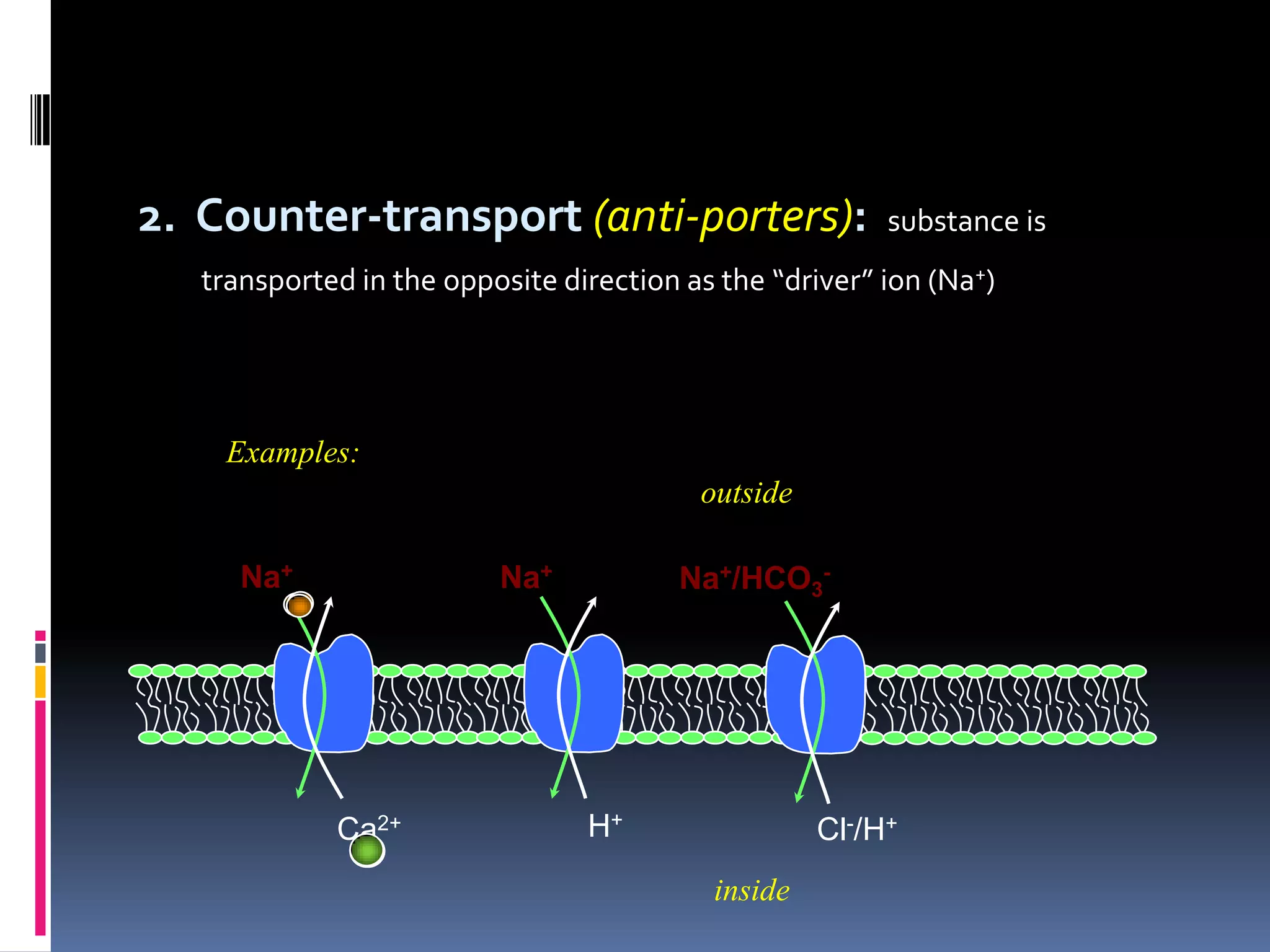
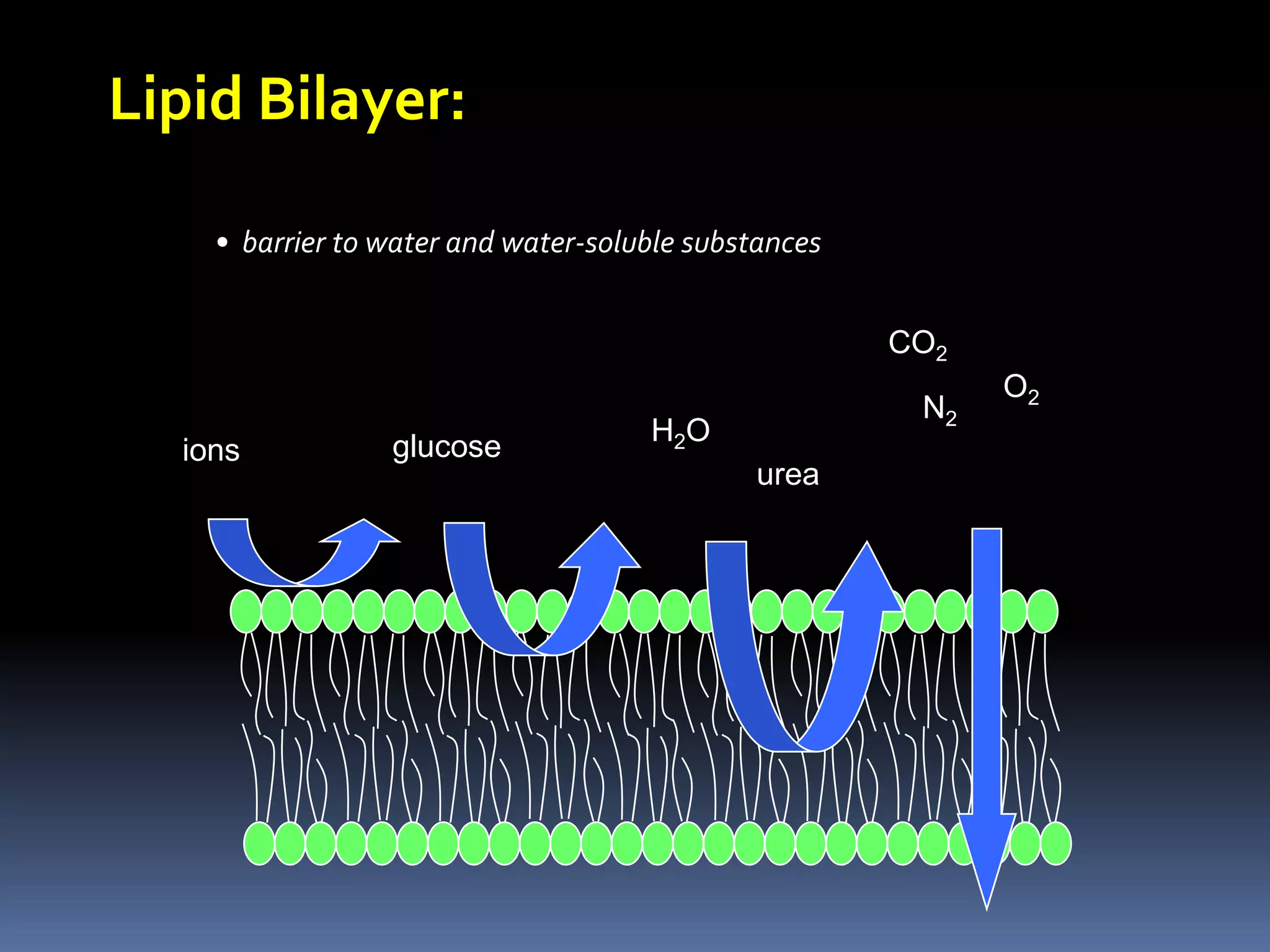
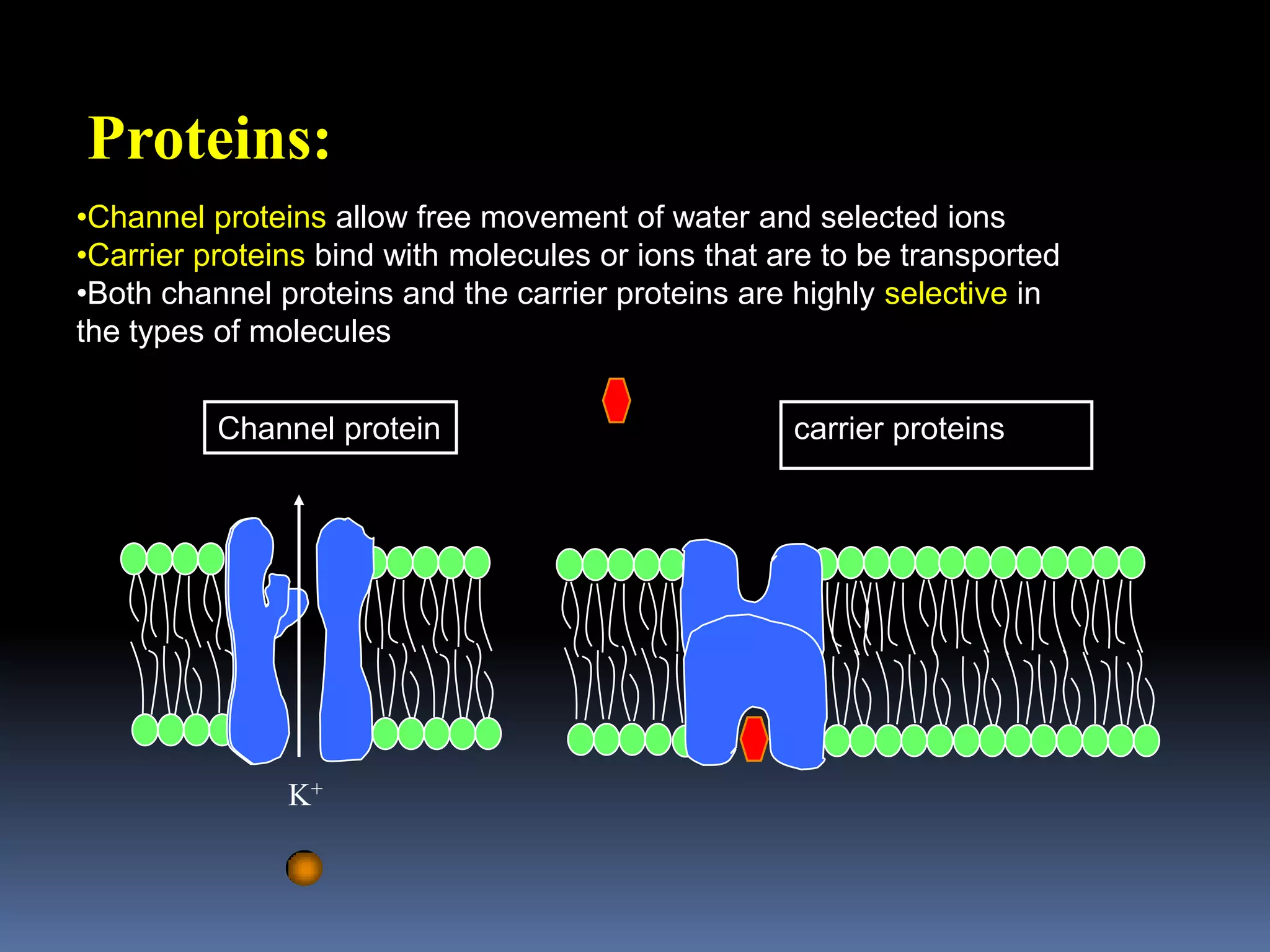
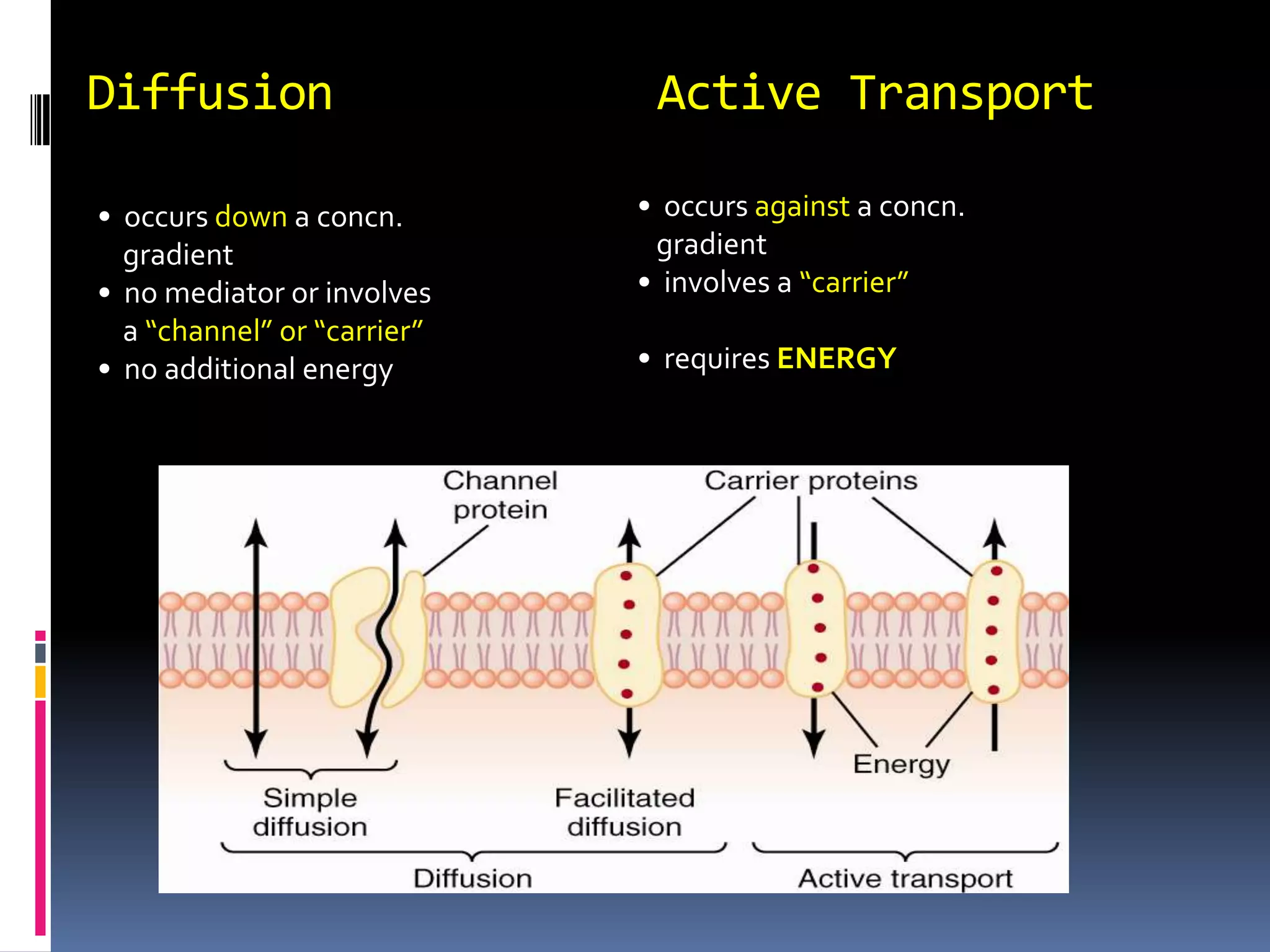
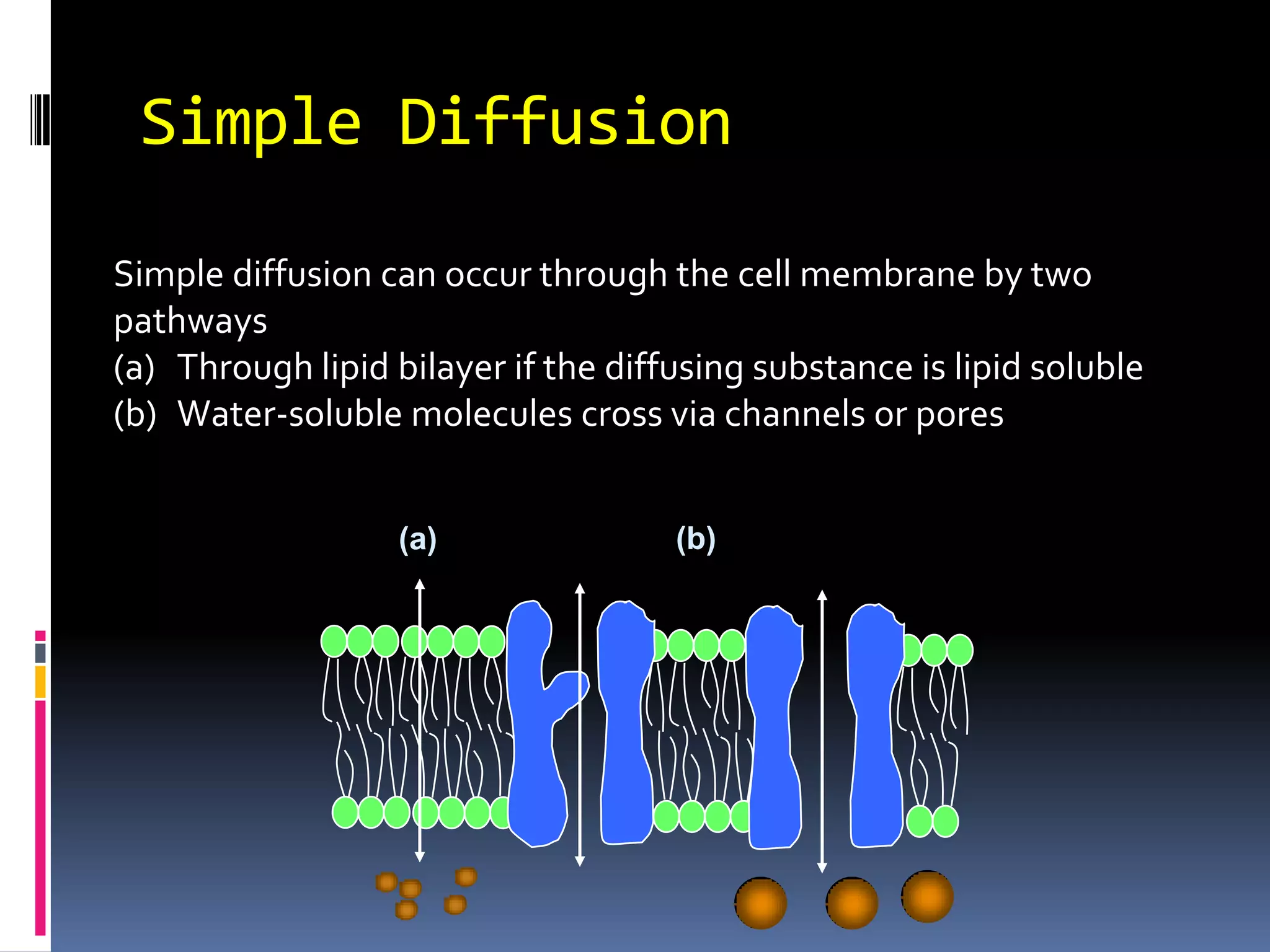
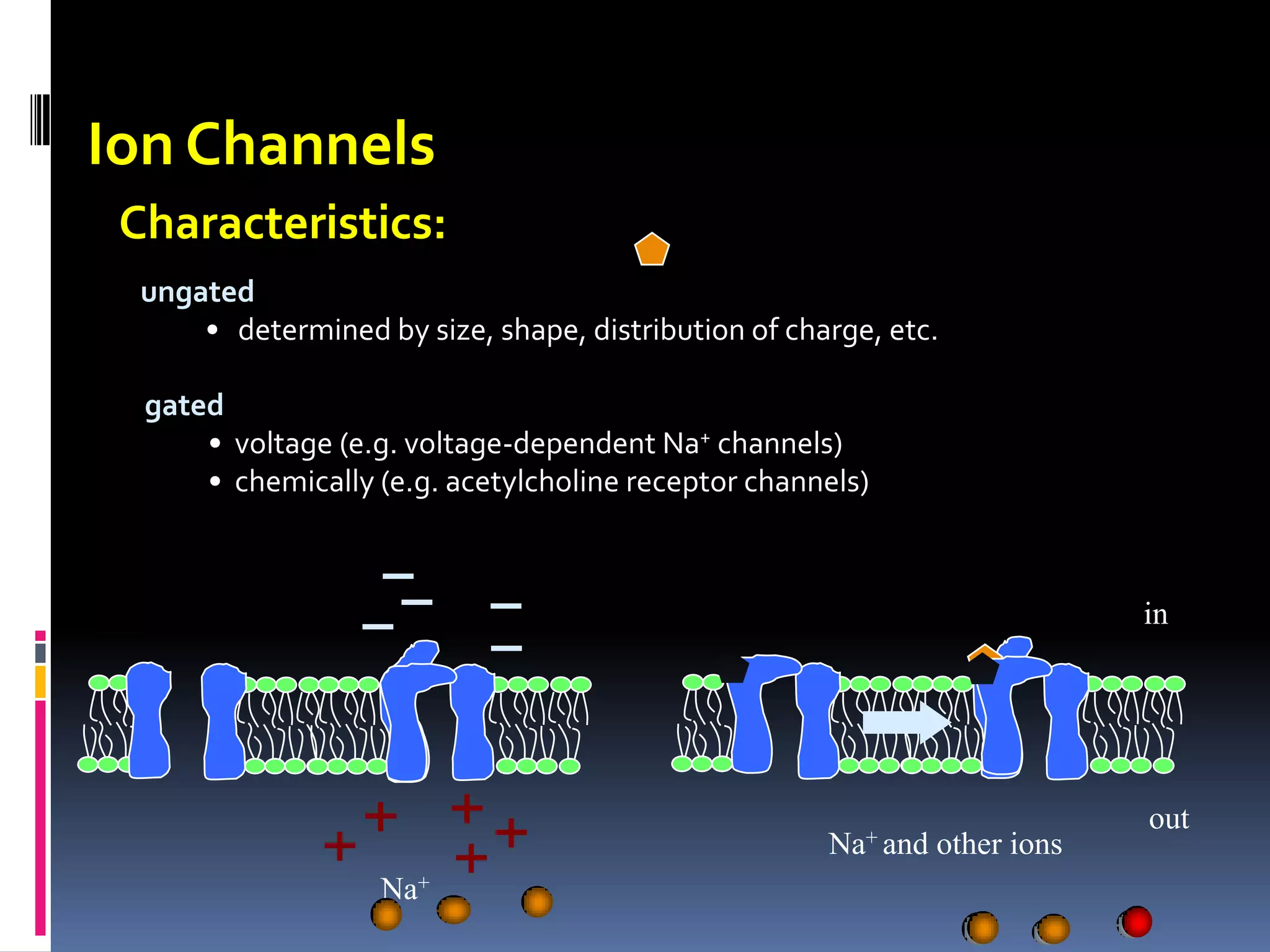
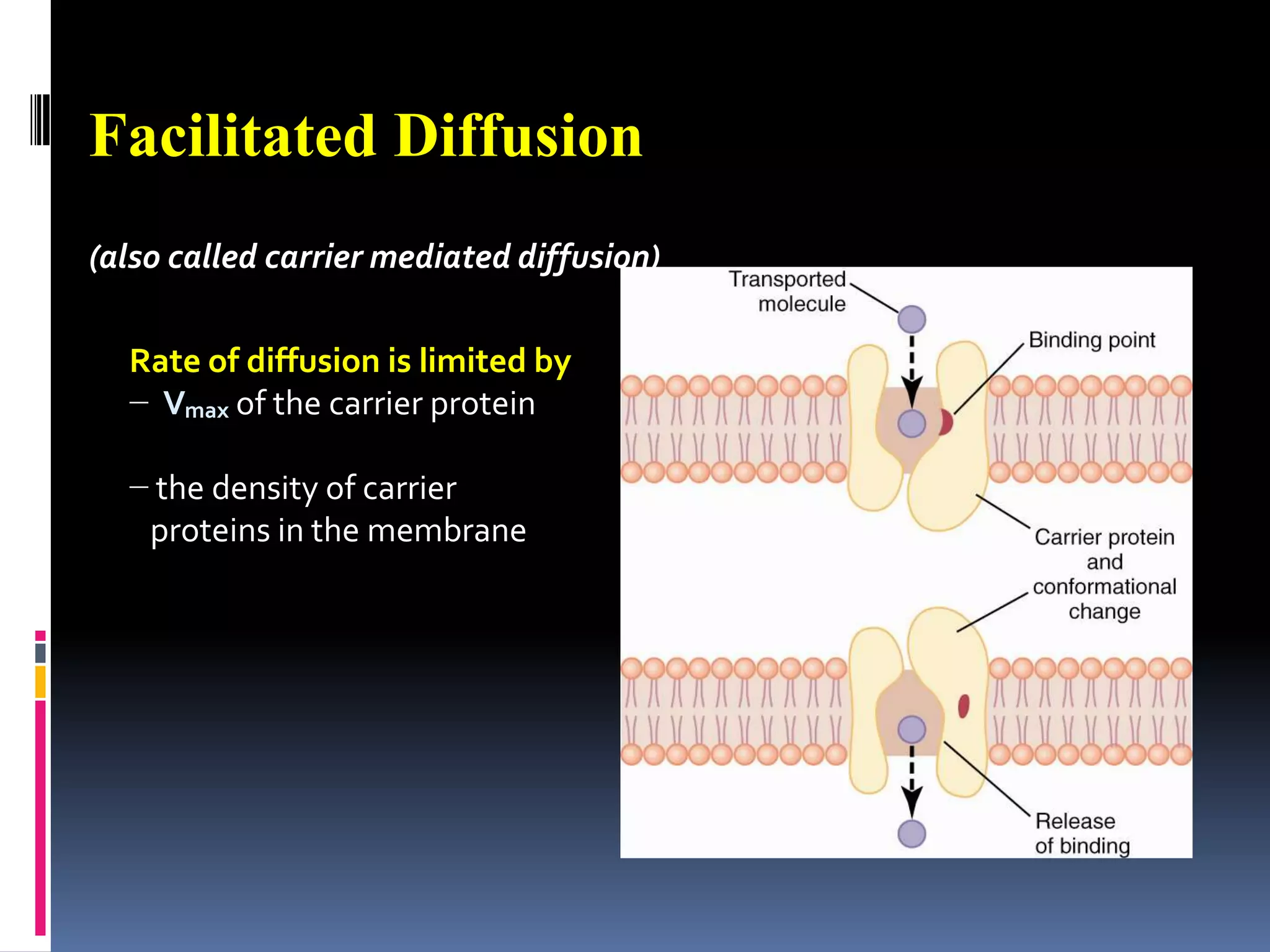
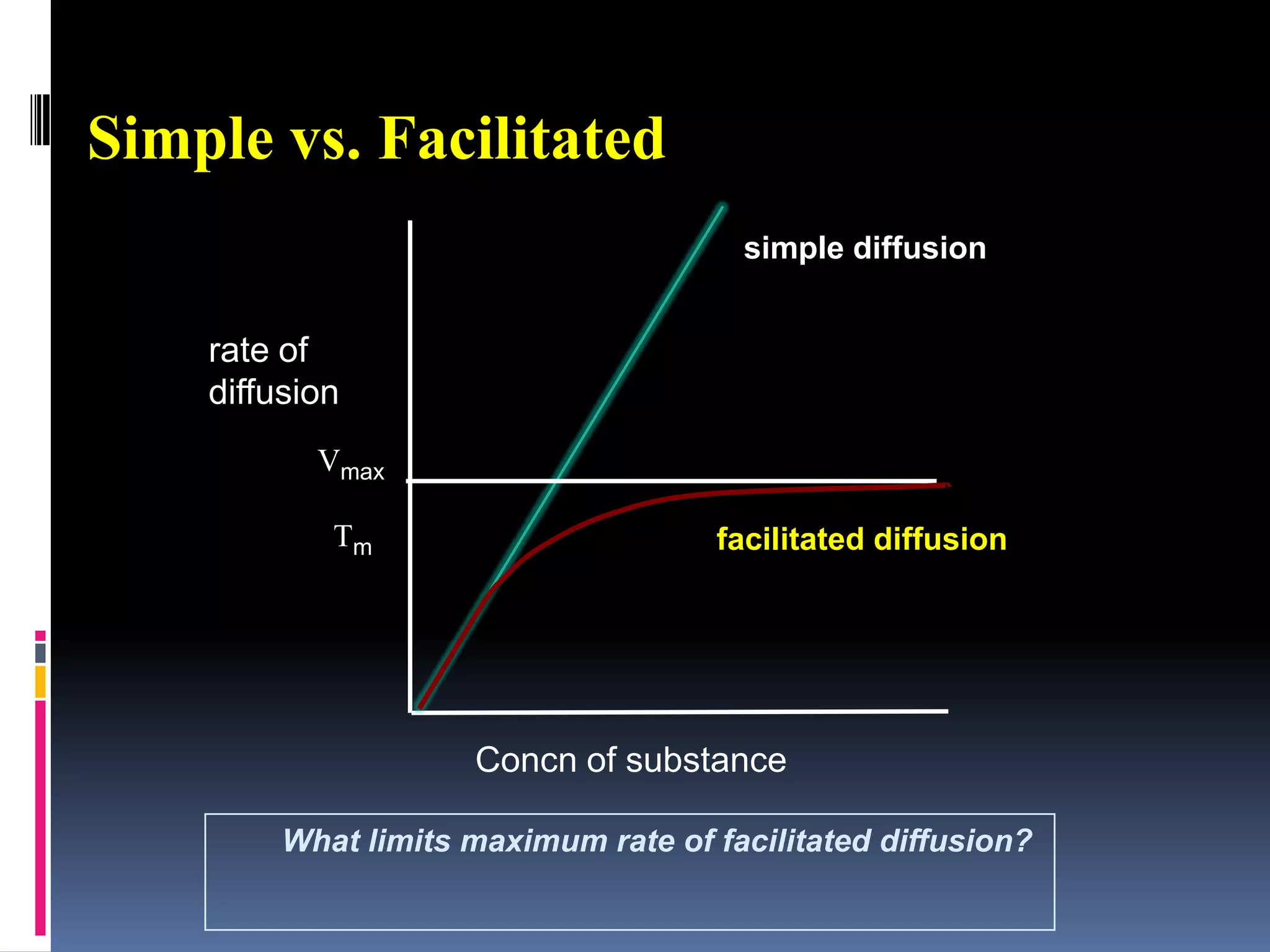
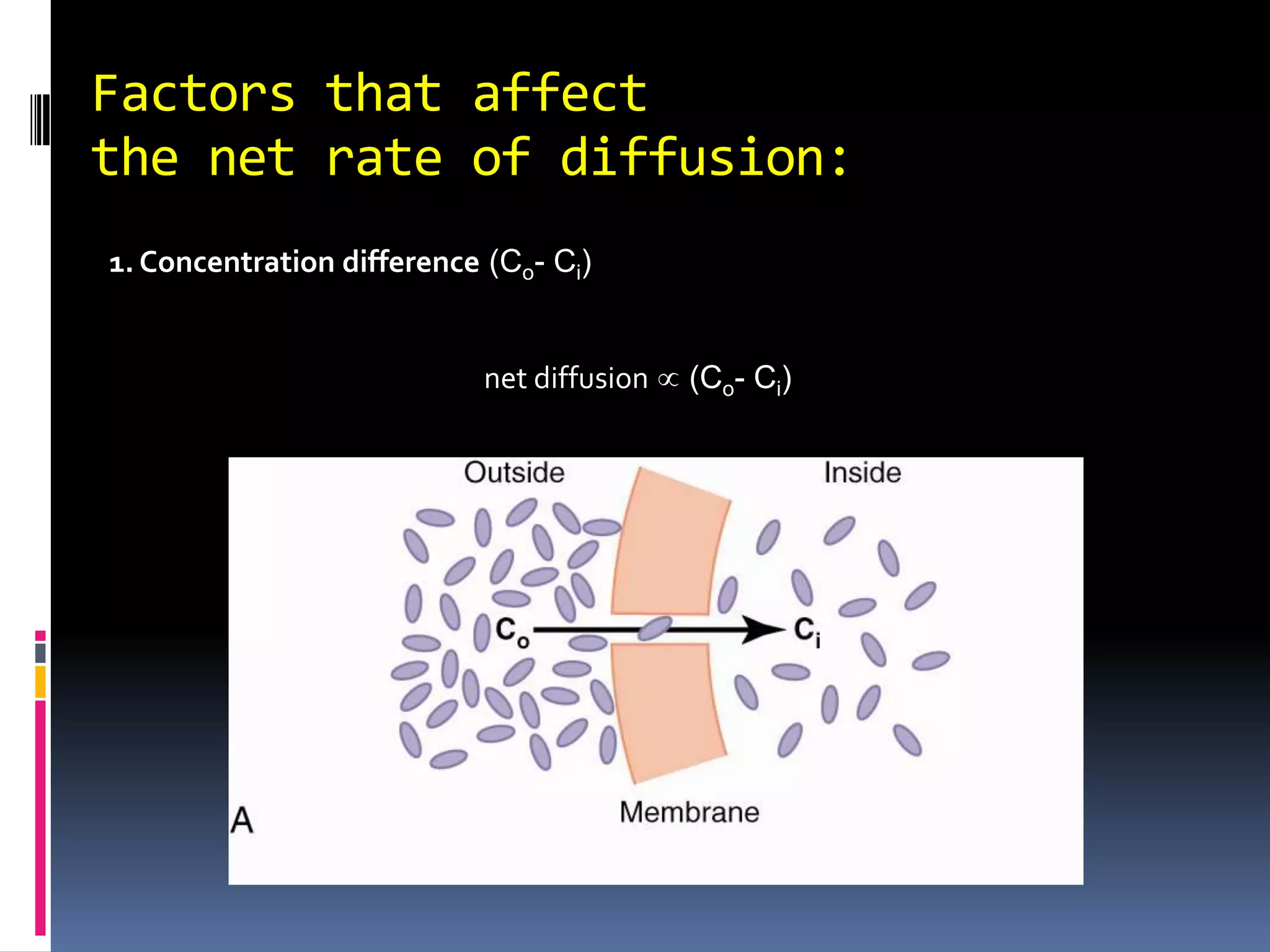
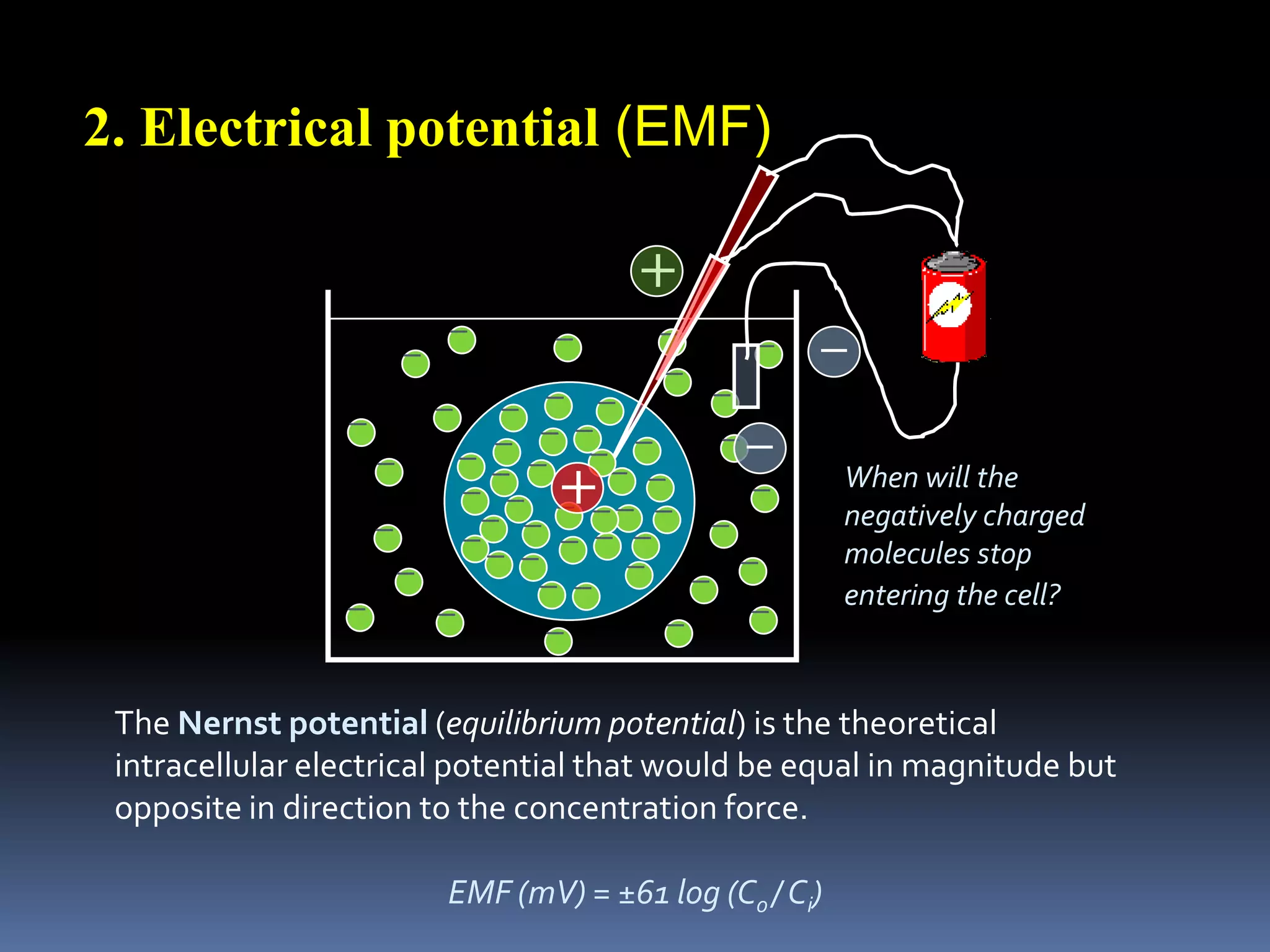
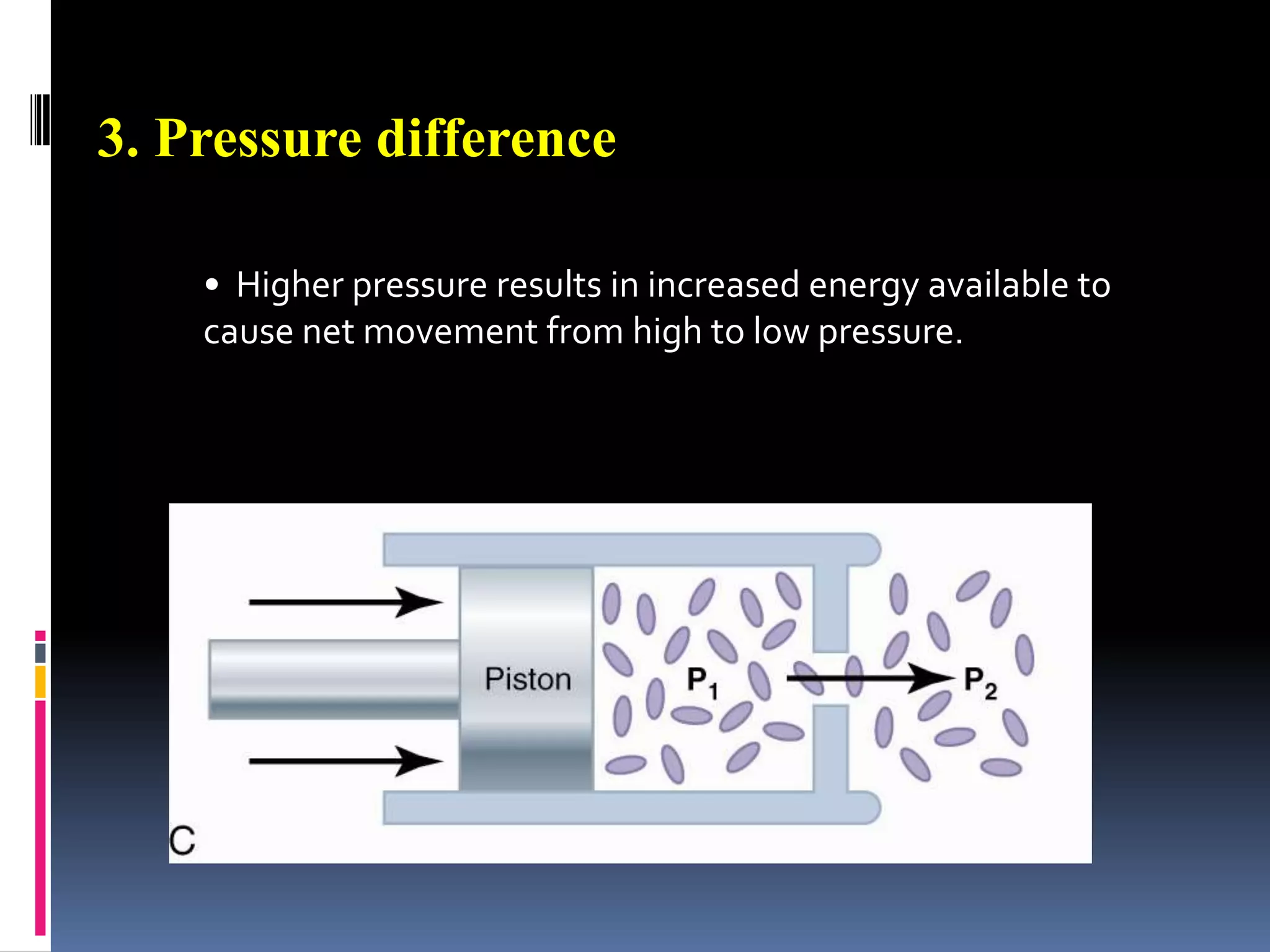
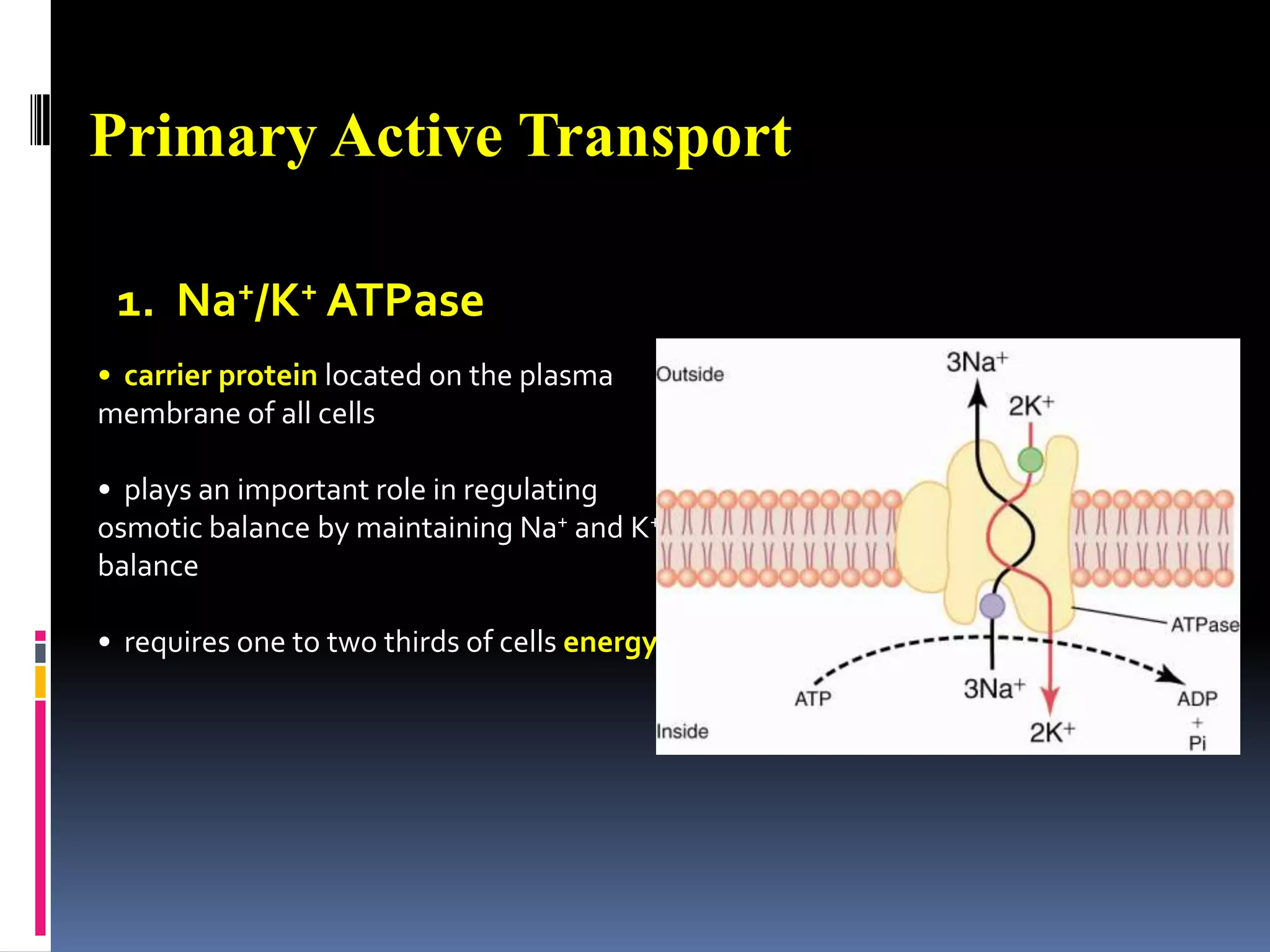
This document discusses transport of substances through the cell membrane. There are molecular gradients that drive diffusion across the membrane. Channel and carrier proteins selectively transport ions and molecules through simple diffusion, facilitated diffusion, or active transport. Primary active transport directly uses ATP to pump molecules against their gradients, while secondary active transport uses the gradient of another molecule like Na+ indirectly.

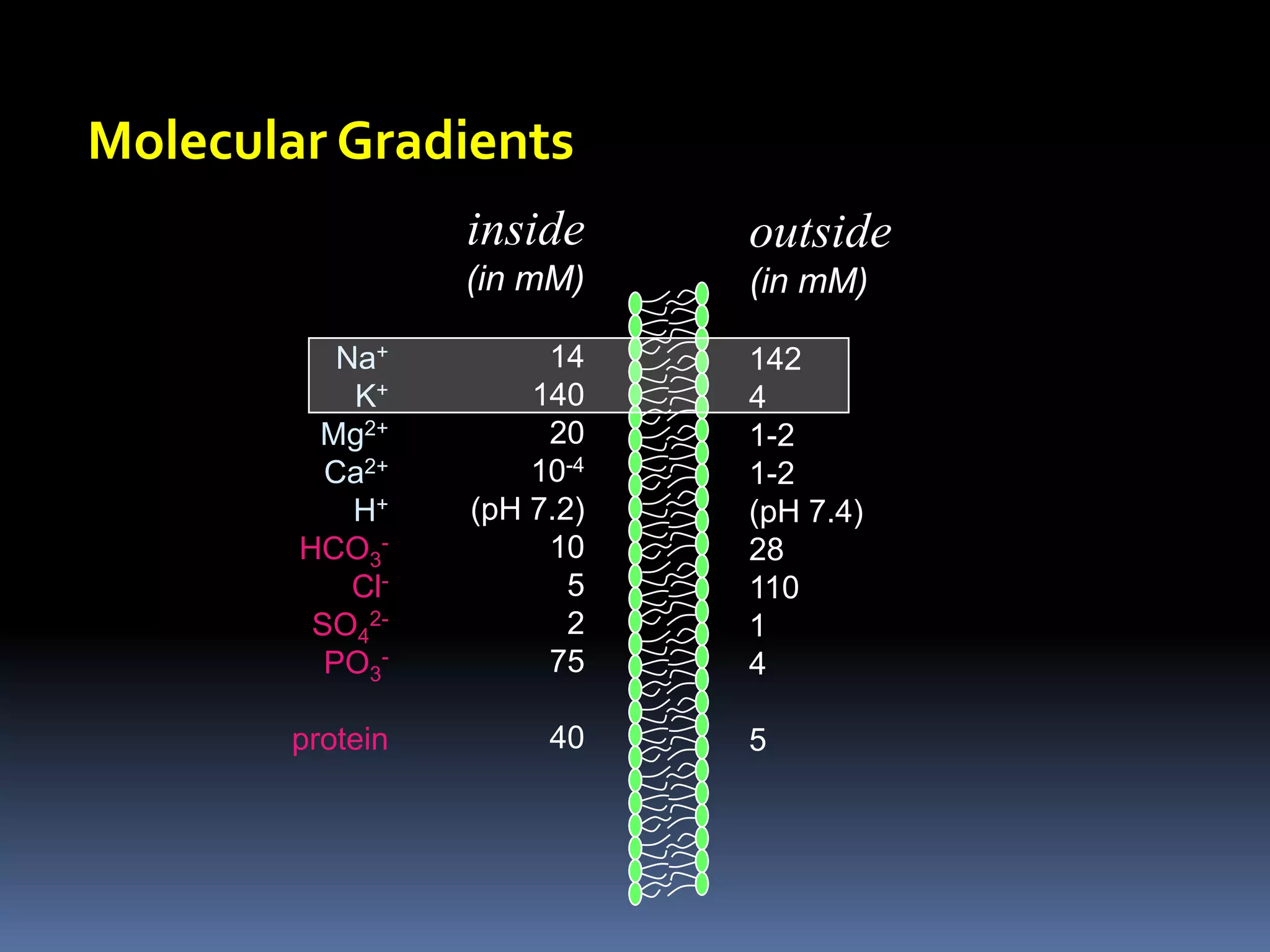
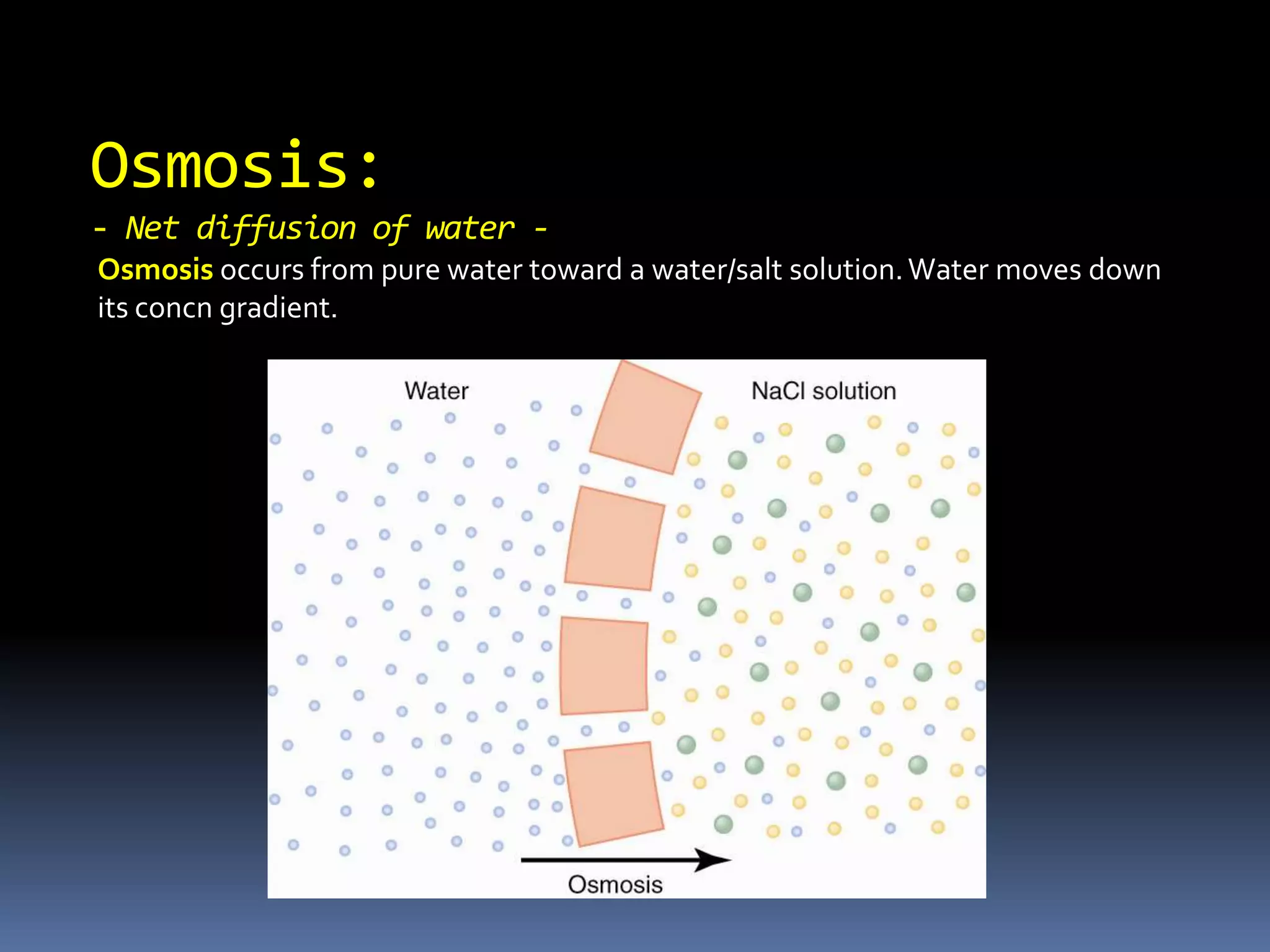
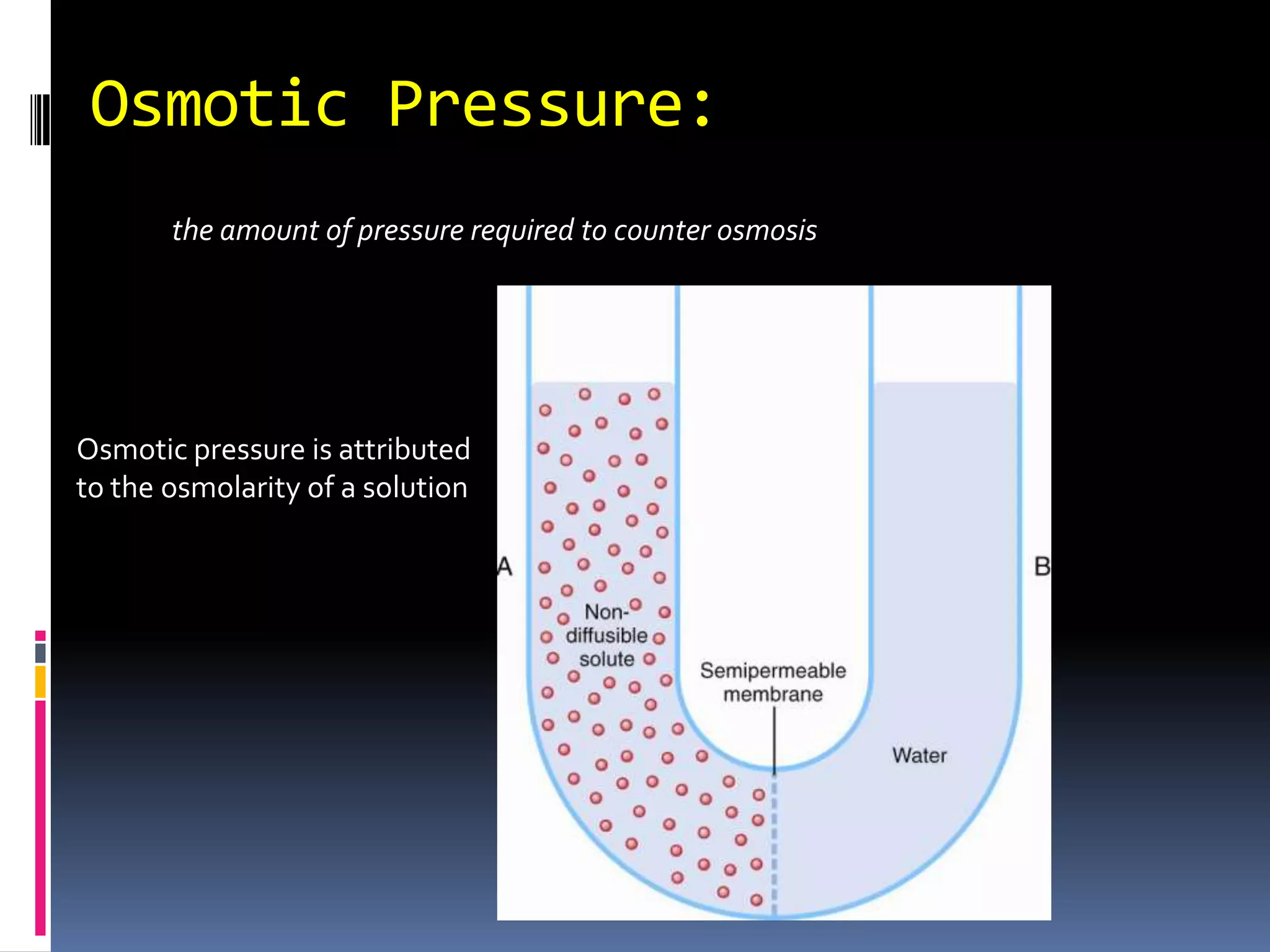
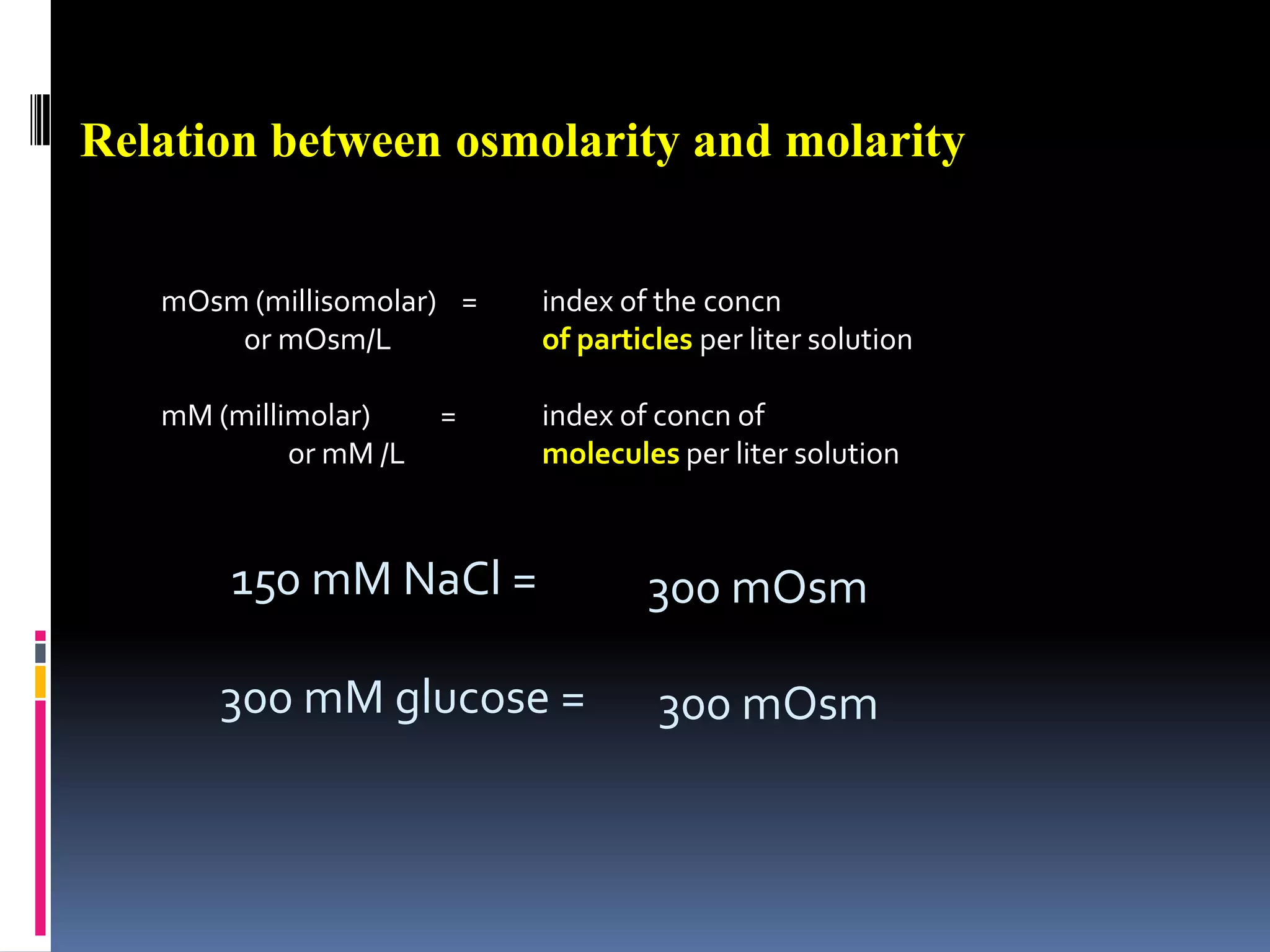
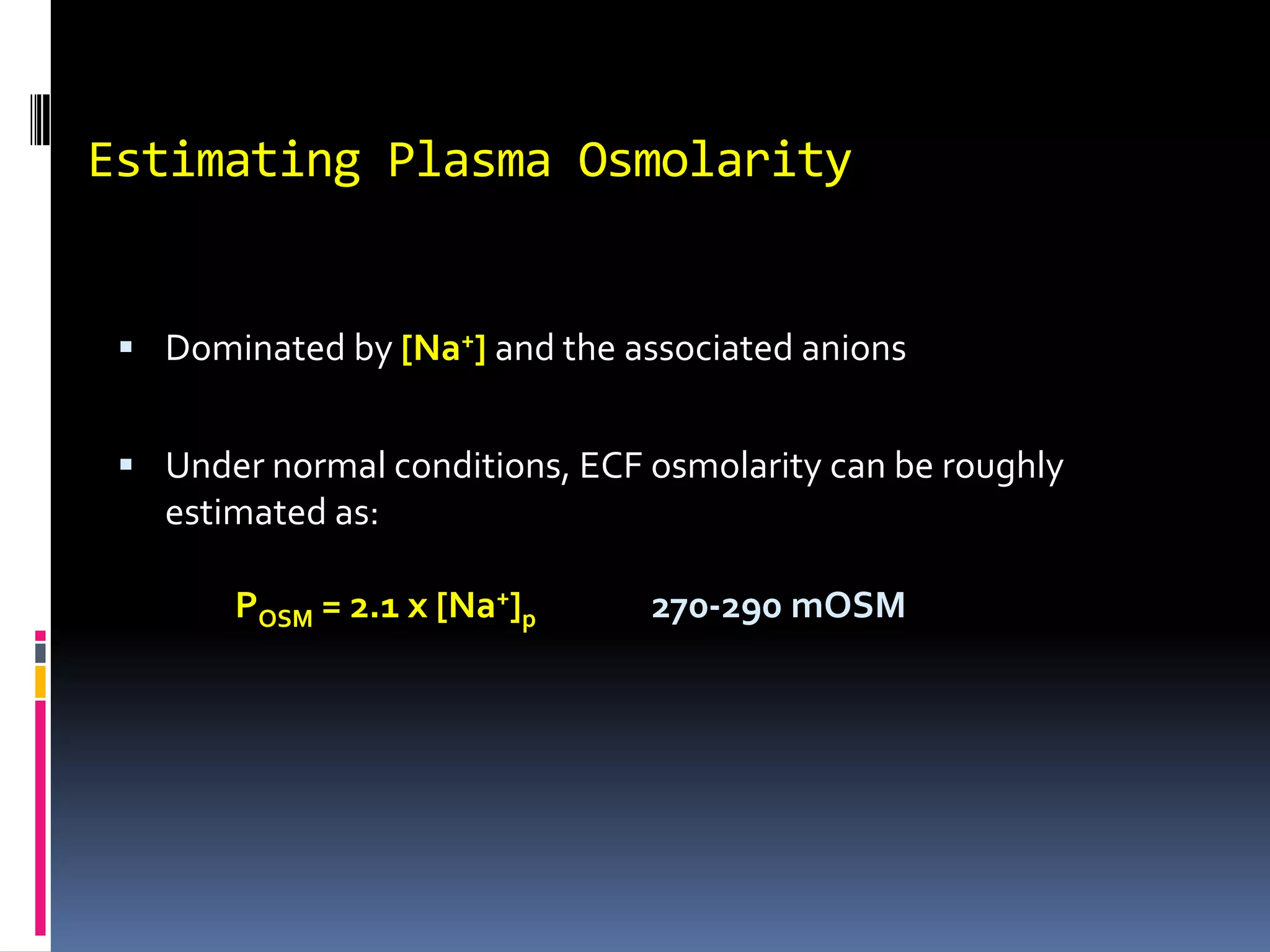


![Estimating Plasma OsmolarityDominated by [Na+]and the associated anionsUnder normal conditions, ECF osmolarity can be roughly estimated as:POSM = 2.1 x [Na+]p270-290 mOSM](https://image.slidesharecdn.com/lec121-10-10-101020230214-phpapp01/75/Lec121-10-10-20-2048.jpg)