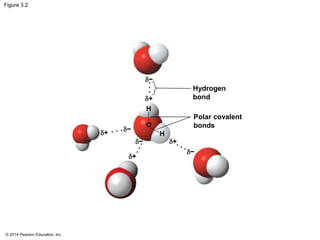
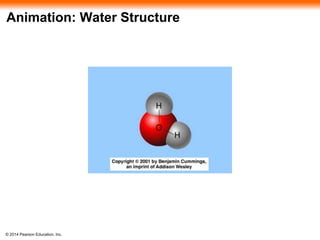
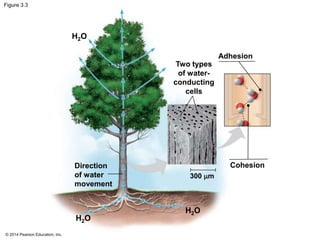
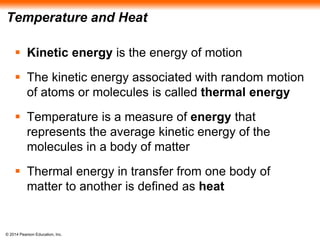
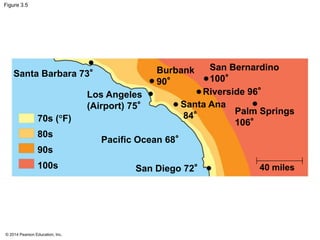
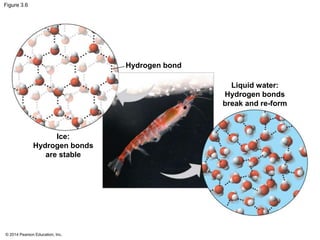
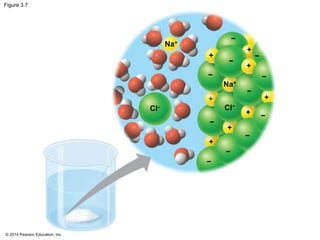
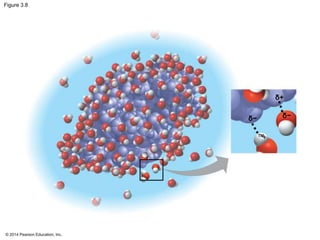
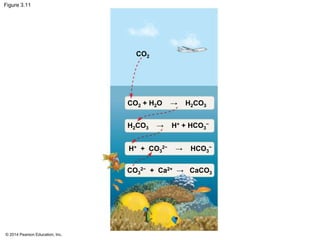
The document summarizes key concepts about water and its importance for life on Earth. It discusses how the polar nature of water molecules allows them to form hydrogen bonds, giving water unique properties like high heat capacity and surface tension. These emergent properties, like moderating temperature fluctuations and enabling solvent properties, help make Earth hospitable for life. The document also explains how water's structure allows transport in organisms and how acids and bases affect hydrogen ion concentration in aqueous solutions.







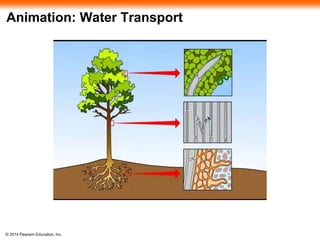
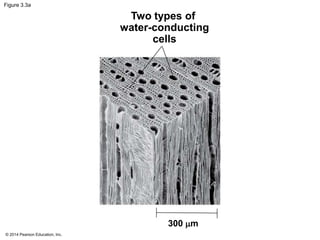

















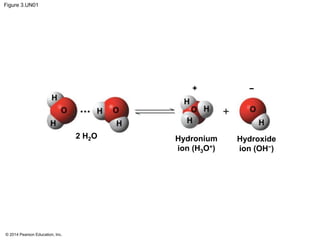


![© 2014 Pearson Education, Inc.
The pH Scale
In any aqueous solution at 25°C the product of H+
and OH− is constant and can be written as
The pH of a solution is defined by the negative
logarithm of H+ concentration, written as
For a neutral aqueous solution, [H+] is 10−7, so
[H+][OH−] = 10− 14
pH = − log [H+]
pH = −(− 7) = 7](https://image.slidesharecdn.com/03lecture-150115095824-conversion-gate01/85/03-Lecture-BIOL-1010-30-Gillette-College-41-320.jpg)

![© 2014 Pearson Education, Inc.
Figure 3.10
H+
H+
H+
H+
H+
H+
H+H+
H+OH–
OH–
OH–
OH–
OH–
OH–
OH–
OH–
H+
H+
H+
H+
H+
H+
OH–
OH–
OH–
OH–
OH–
OH–
Basic
solution
Neutral
solution
Acidic
solution
IncreasinglyAcidic
[H+]>[OH−]
Neutral
[H+] = [OH−]
pH Scale
IncreasinglyBasic
[H+]<[OH−]
Battery acid
Gastric juice, lemon juice
Vinegar, wine,
cola
Tomato juice
Beer
Black coffee
Rainwater
Pure water
Human blood, tears
Seawater
Inside of small intestine
Urine
Saliva
Milk of magnesia
Household ammonia
Household
bleach
Oven cleaner
0
1
2
3
4
5
6
7
8
9
10
11
12
13
14](https://image.slidesharecdn.com/03lecture-150115095824-conversion-gate01/85/03-Lecture-BIOL-1010-30-Gillette-College-43-320.jpg)


![© 2014 Pearson Education, Inc.
Figure 3.UN02a
Calcificationrate
(mmolCaCO3/m2⋅day)
[CO3
2−] (μmol/kg of seawater)
20
10
0
220 240 260 280](https://image.slidesharecdn.com/03lecture-150115095824-conversion-gate01/85/03-Lecture-BIOL-1010-30-Gillette-College-49-320.jpg)

![© 2014 Pearson Education, Inc.
Figure 3.UN04
Acidic
[H+] > [OH−]
Neutral
[H+] = [OH−]
Basic
[H+] < [OH−]
Acids donate H+ in
aqueous solutions.
Bases donate OH−
or accept H+ in
aqueous solutions.
0
7
14](https://image.slidesharecdn.com/03lecture-150115095824-conversion-gate01/85/03-Lecture-BIOL-1010-30-Gillette-College-52-320.jpg)
