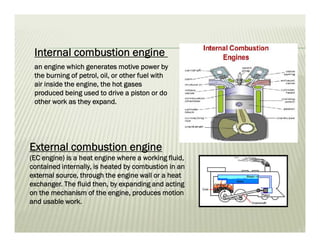
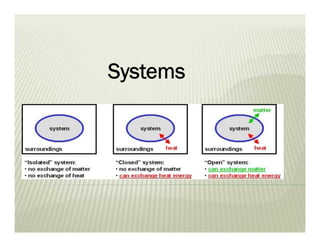
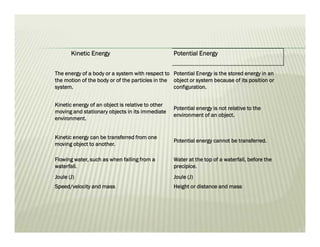
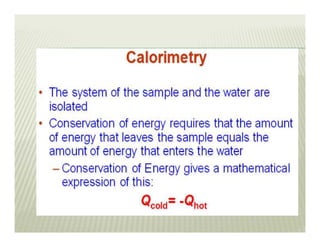
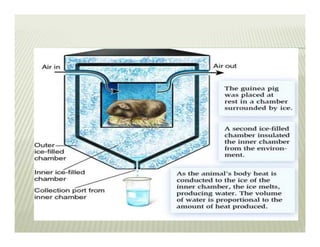
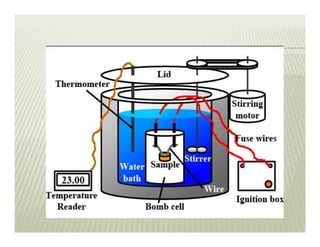
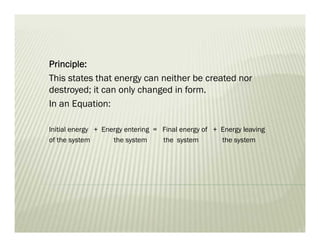
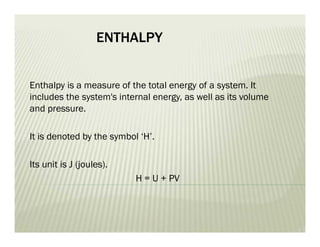
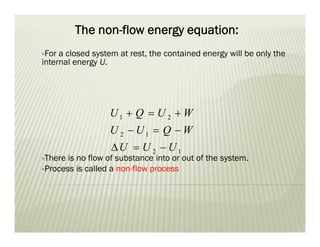
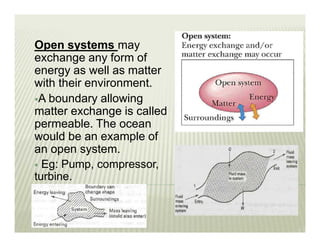
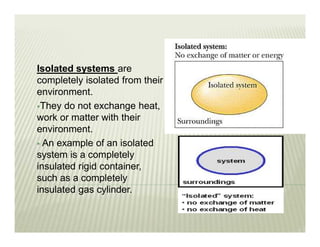
This document discusses various thermodynamic concepts and processes. It defines adiabatic processes as those where no heat is transferred into or out of the system. It also discusses liquefaction of gases which involves increasing pressure on a gas to bring its molecules closer together and reduce its temperature enough to change it from a gas to a liquid. Finally, it defines different types of engines - external combustion engines where combustion occurs outside the engine and heats a working fluid, and internal combustion engines where fuel combusts inside the engine.