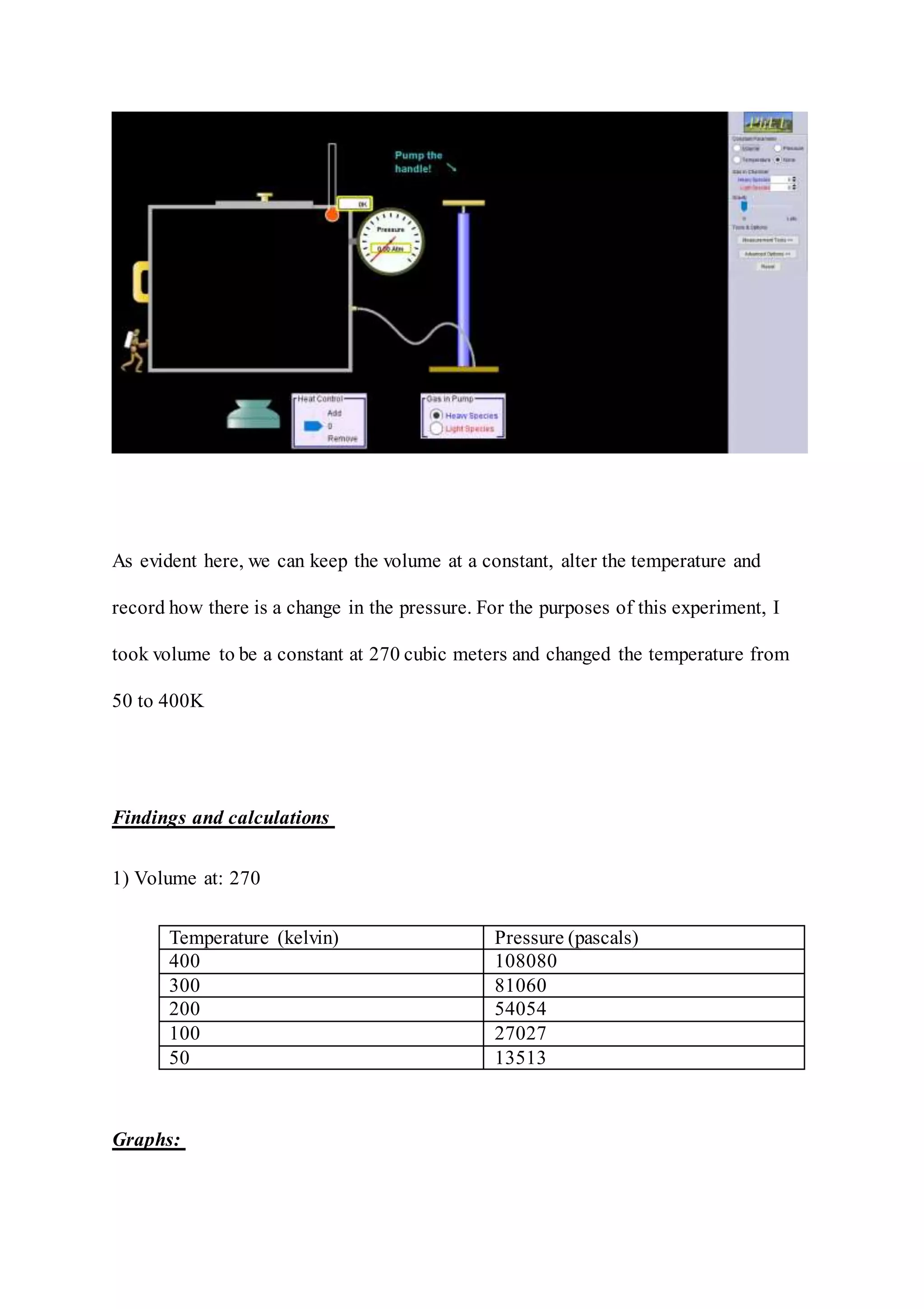
The document summarizes an experiment to examine the relationship between pressure and temperature as described by Gay-Lussac's law. The experiment was simulated using an online simulation program. The simulation showed that as temperature increased from 50K to 400K at a constant volume of 270 cubic meters, the pressure also increased in a linear fashion from 13513 pascals to 108080 pascals, validating the direct proportionality predicted by Gay-Lussac's law. However, the document notes that a real-world experiment may yield different results than a simulation due to gases not being truly perfect.