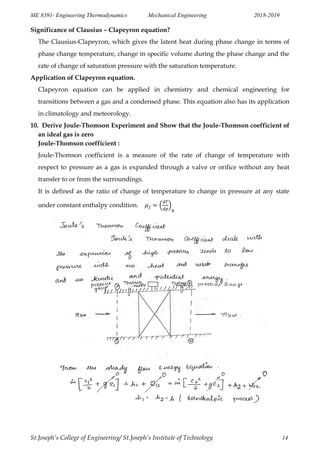
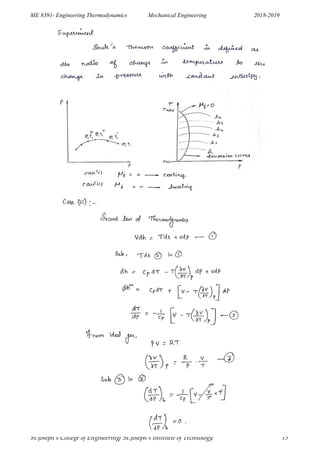
This document outlines the key concepts in ideal and real gases covered in the Engineering Thermodynamics course ME 8391. It defines ideal and real gases, equations of state, reduced properties, assumptions in the van der Waals equation of state, Avogadro's law, coefficients of volume expansion, the Helmholtz function, compressibility factor, laws of corresponding states for gases, Boyle's law, Charles' law, and more. It also provides sample problems and derivations related to Maxwell relations, entropy equations, heat capacities, Clausius-Clapeyron equation, Joule-Thomson effect, and inversion temperature.

![ME 8391- Engineering Thermodynamics Mechanical Engineering 2018-2019
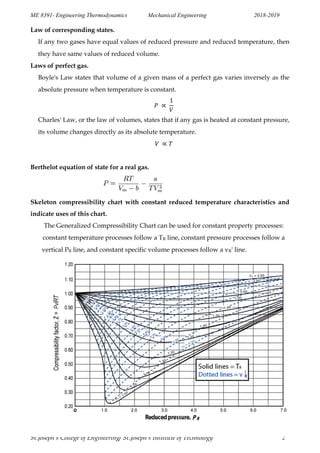
St.Joseph’s College of Engineering/ St.Joseph’s Institute of Technology 3
Bulk Modulus
The bulk modulus (K) of a substance is defined as the ratio between the increase in
pressure and the corresponding decrease in volume as a fraction of its original volume
under isothermal condition.
( ) ( ) ( )
Isothermal compressibility
The isothermal compressibility (KT) of a substance is defined as ratio between the
reduction in volume as a fraction of its original volume and the corresponding increase
in pressure under isothermal condition.
( ) ( )
Isentropic compressibility
The isentropic compressibility (Ks) of a substance is defined as ratio between the
reduction in volume as a fraction of its original volume and the corresponding increase
in pressure under isentropic condition.
( ) ( )
Coefficient of thermal expansion?
The coefficient of expansion of a substance is defined as ratio between the increase in
volume as a fraction of its original volume and the corresponding increase in
temperature under isobaric condition.
( ) ( )
How does the Vander Waal’s equation differ from the ideal gas equation of state?
The ideal gas equation pV=mRT has two important assumptions,
There is little or no attraction between the molecules of the gas.
The volume occupied by the molecules themselves is negligibly small compared to
the volume of the gas.
This equation holds good for low pressure and high temperature ranges as the
intermolecular attraction and the volume of the molecules are not of much
significance. As the pressure increases, inter molecular forces of attraction and
repulsion increases and the volume of the molecules are not negligible. The real gas
deviates considerably from the ideal gas equation [p+ (a/V2)] (V-b) = RT]](https://image.slidesharecdn.com/me8391engineeringthermodynamicsunitiv-210908044024/85/Me8391-engineering-thermodynamics-unit-iv-3-320.jpg)