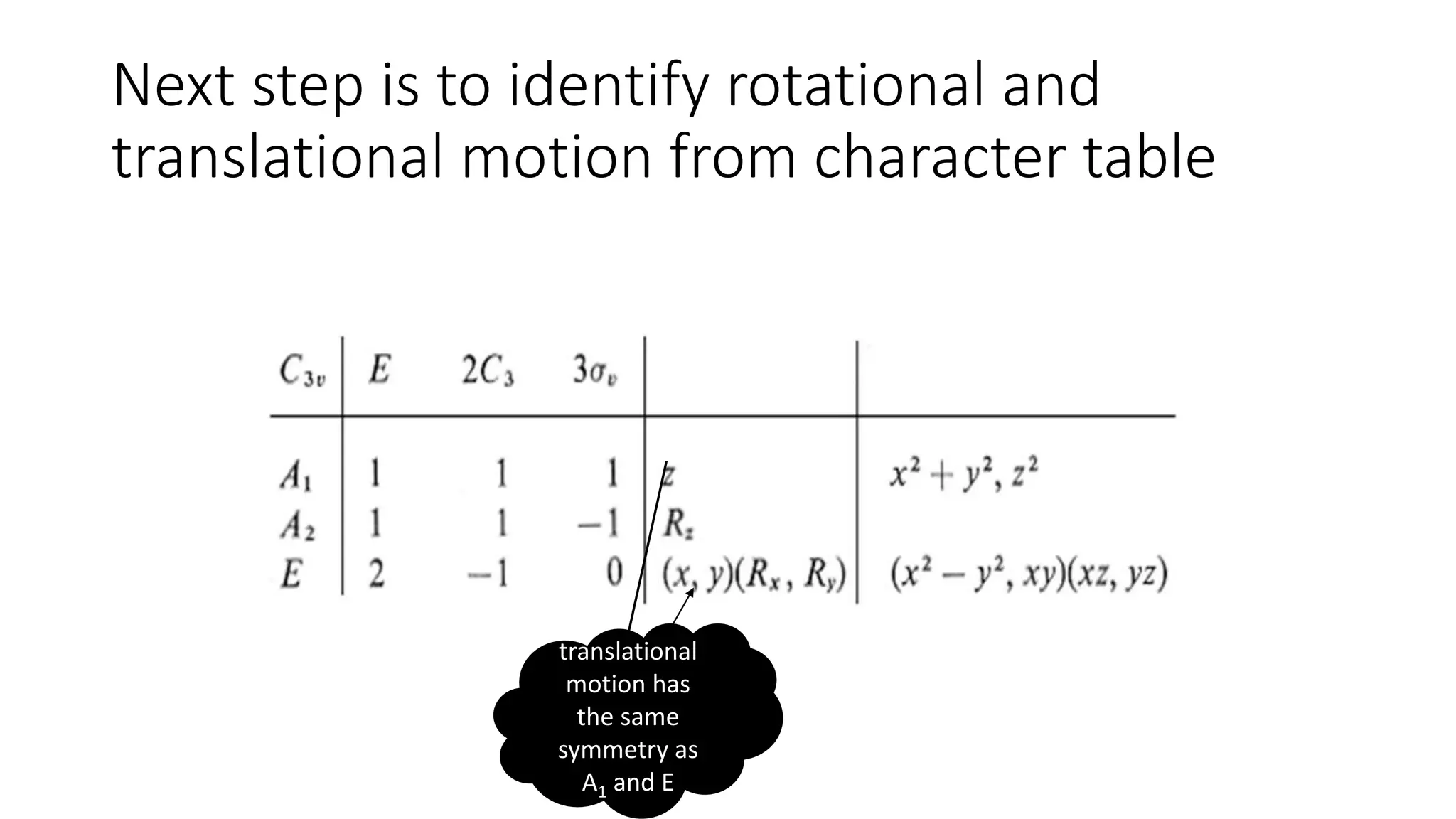
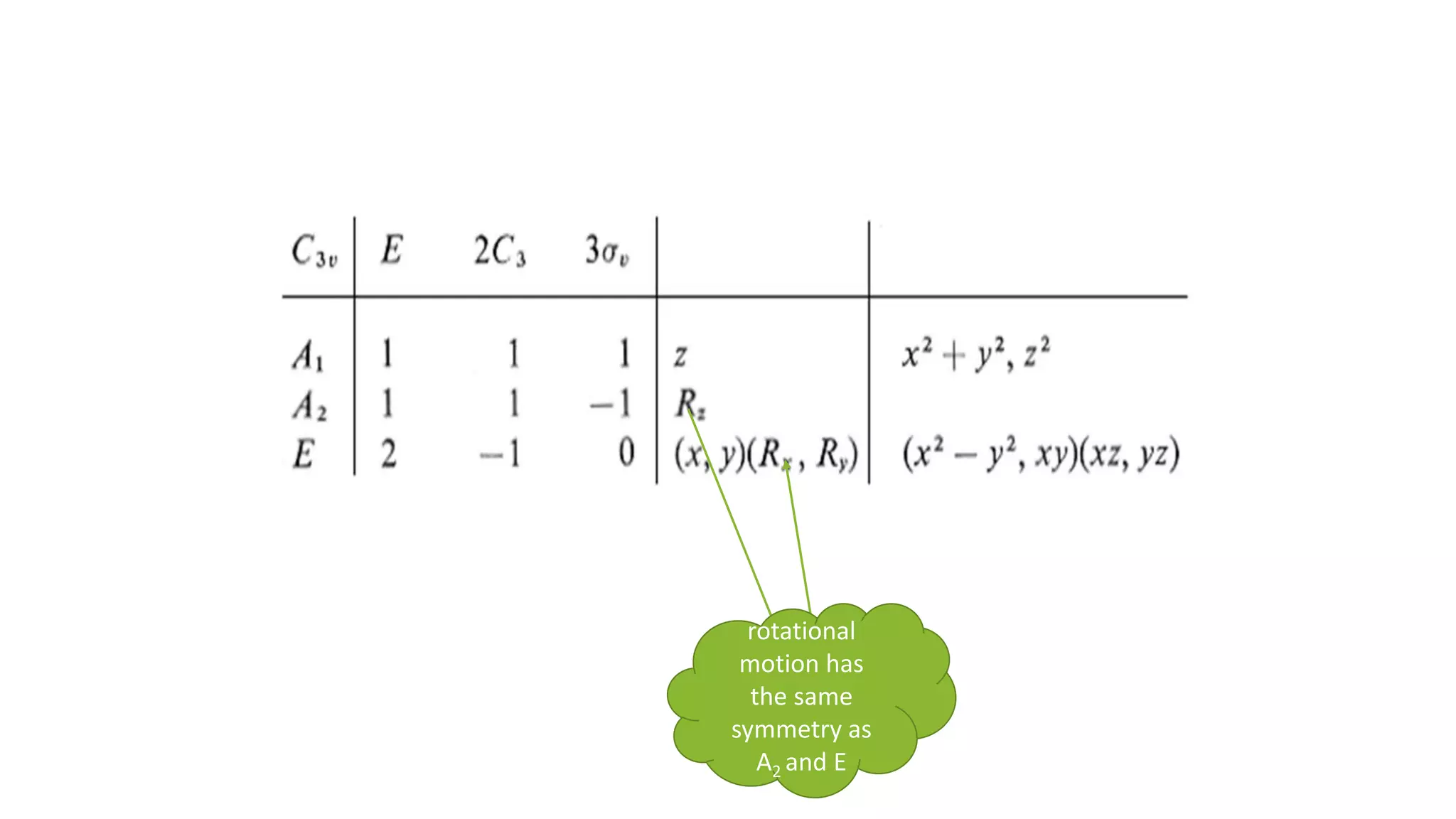
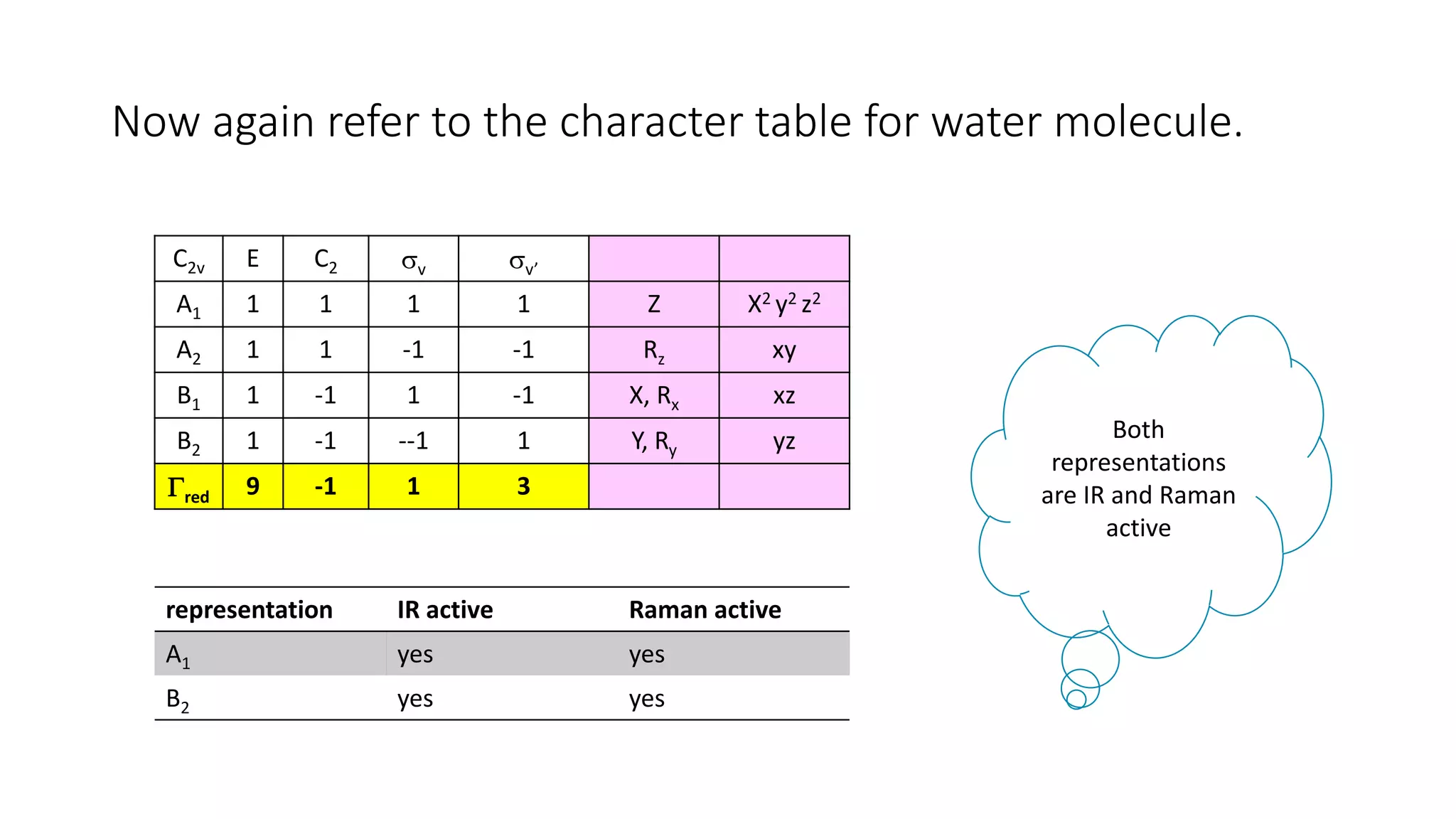
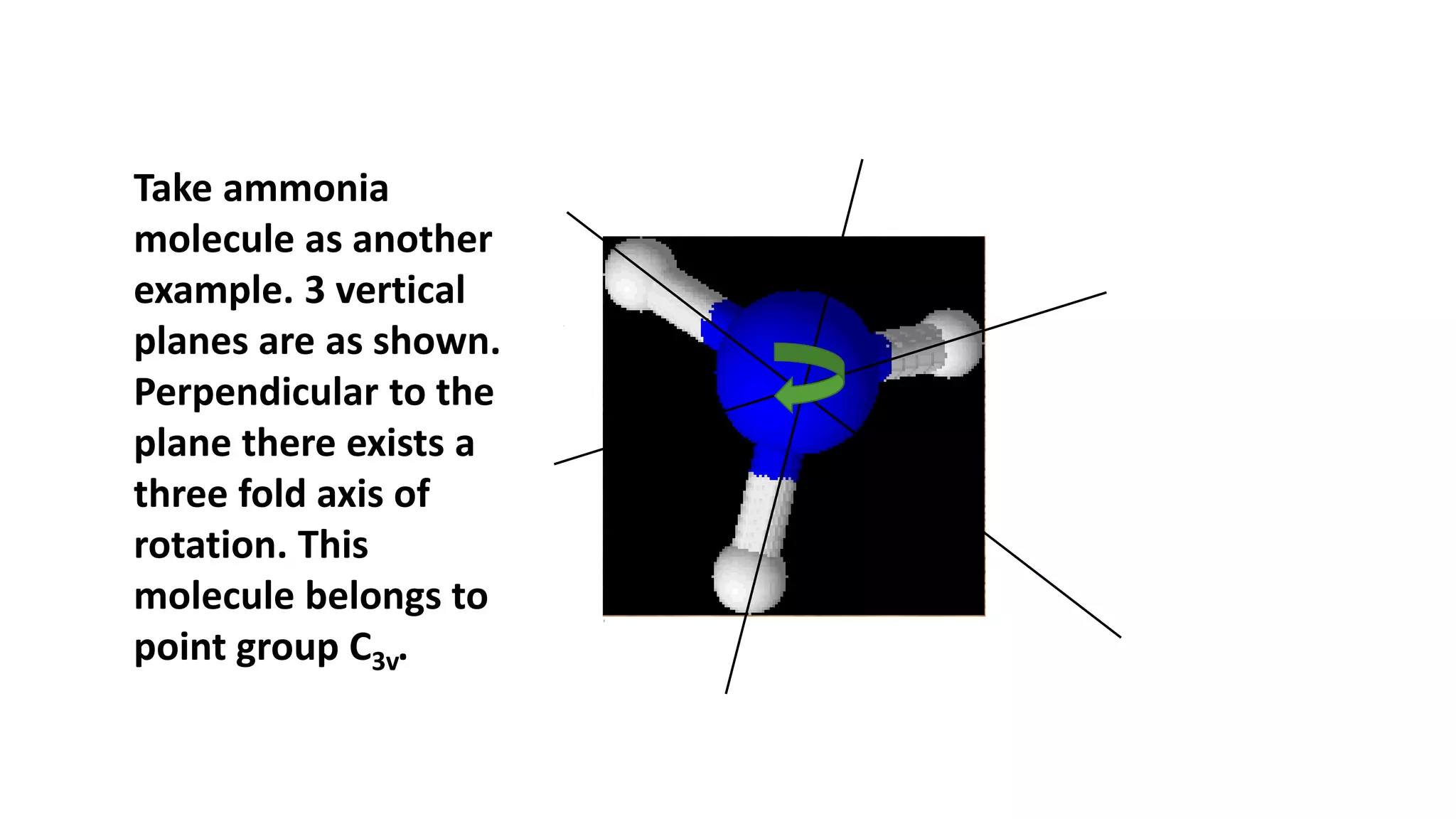
The document outlines how to read a character table in vibrational spectroscopy, detailing the process of utilizing group theory to analyze molecular vibrations and their associated infrared (IR) and Raman activities. It explains the steps needed to identify a molecule's point group and decomposes the reducible representations into irreducible ones using a character table, with specific examples provided using the water molecule. The document also addresses the predictions of IR and Raman active vibrations based on changes in dipole moment and polarizability.





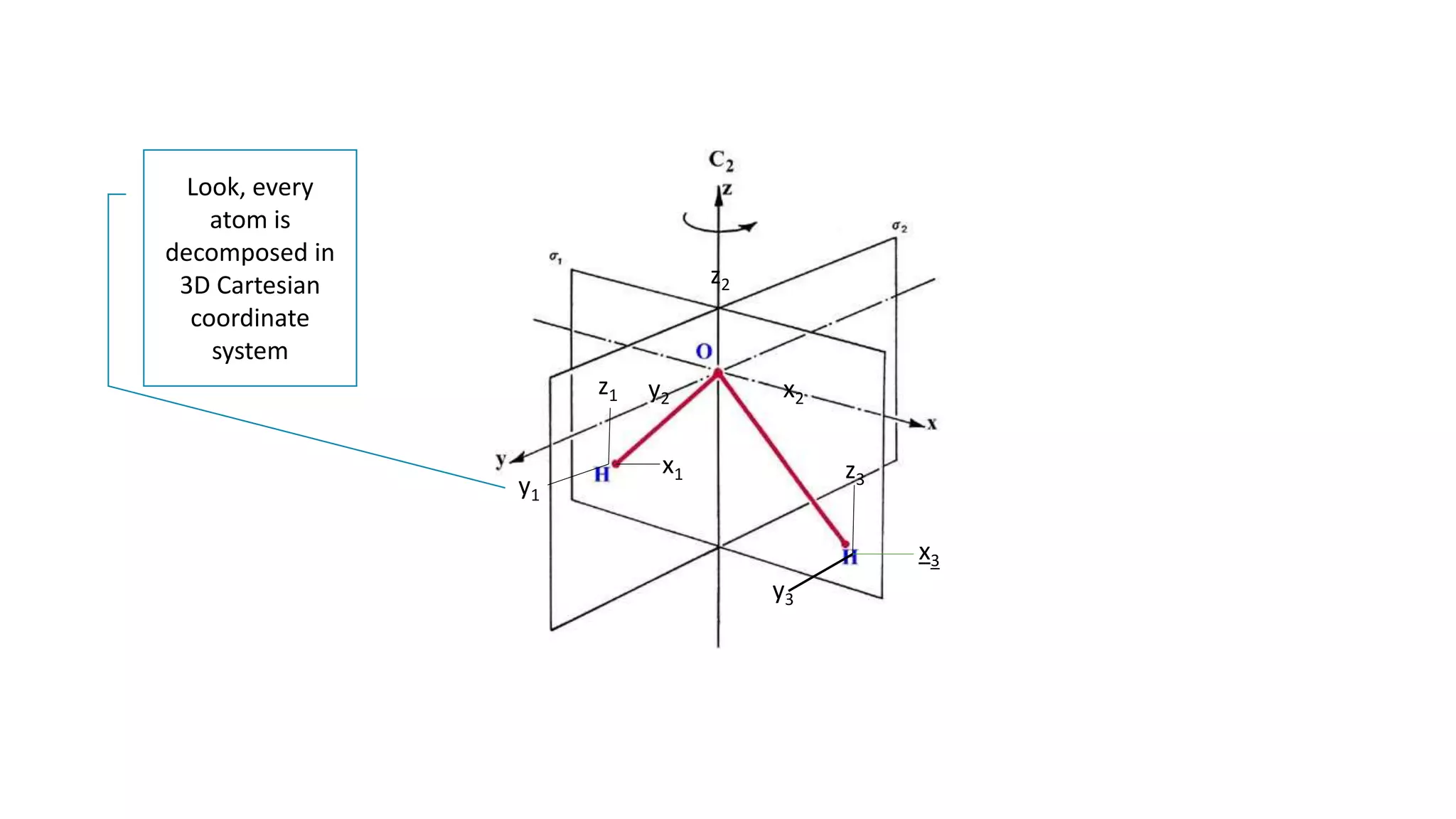








![This way, the characters obtained for water molecule belongs to
a reducible representation, if every atom is given a 3D basis.
C2v E C2 v v’
A1 1 1 1 1
A2 1 1 -1 -1
B1 1 -1 1 -1
B2 1 -1 --1 1
red 9 -1 1 3
ai= i/h [ I (R)*j(R) ]
Use standard reduction formula
a1= ¼[ 9*1*1+(-1)*1*1+1*1*1+3*1*1]=1/4[9-1+1+3]=1/4*12=3
a2= ¼[ 9*1*1+(-1)*1*1+1*-1*1+3*-1*1]=1/4[9-1-1-3]=1/4*4=1
b1= ¼[ 9*1*1+(-1)*1*1+1*-1*1+3*1*1]=1/4[9+1+1-3]=1/4*8=2
b2= ¼[ 9*1*1+(-1)*-1*1+1*-1*1+3*-1*1]=1/4[9+1-1+3]=1/4*12=3
Clearly
red = 3A1 +A2 +2B1 + 3B2](https://image.slidesharecdn.com/vibrationalsp-170623143521/75/character-table-in-vibrational-spectroscopy-16-2048.jpg)

















![Next we have to decompose reducible
representations as the sum of irreducible rep.
• A1 = 1/6[12*1*1 + 0*1*2 + 2*1*3]= 1/6[12+0+6]= 3
• A2 = 1/6[12*1*1 + 0*-1*2 +2*-1*3]= 1/6[12+0-6]= 1
• E = 1/6[12*2 +0*0*2 + 2*0*3] =1/6[24+0+0]=4
• Final solution is
• red = 3A1+A2+4E How can we see that
this solution has no
computational error?](https://image.slidesharecdn.com/vibrationalsp-170623143521/75/character-table-in-vibrational-spectroscopy-37-2048.jpg)