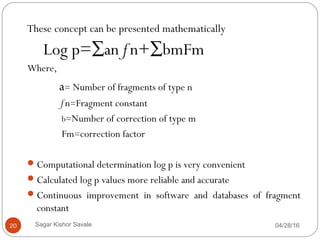
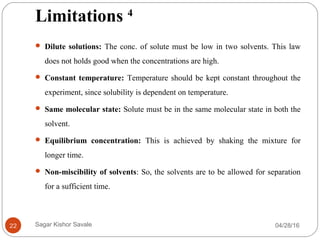
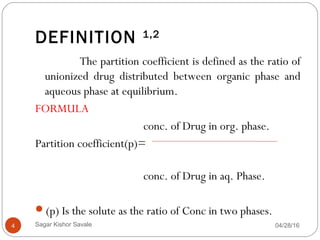
This document discusses the concept of partitioning, specifically the partition coefficient which defines the distribution of a solute between two immiscible phases at equilibrium. It outlines methods for determining partition coefficients, such as the shake flask method, HPLC, and computational approaches, while also discussing the implications of log P values on drug solubility, absorption, and distribution. The document includes general features, limitations of the determination methods, and references for further reading.



![04/28/1611
Where,
[Drug molecule]o = concentration of drug in its molecular form in octan-1-ol;
[Drug molecule]w = concentration of drug in its molecular form in water;
[Drug ion]w =concentration of drug in its ionised form in water.
Sagar Kishor Savale](https://image.slidesharecdn.com/partitioncoefficient-160428054510/85/Partition-coefficient-11-320.jpg)