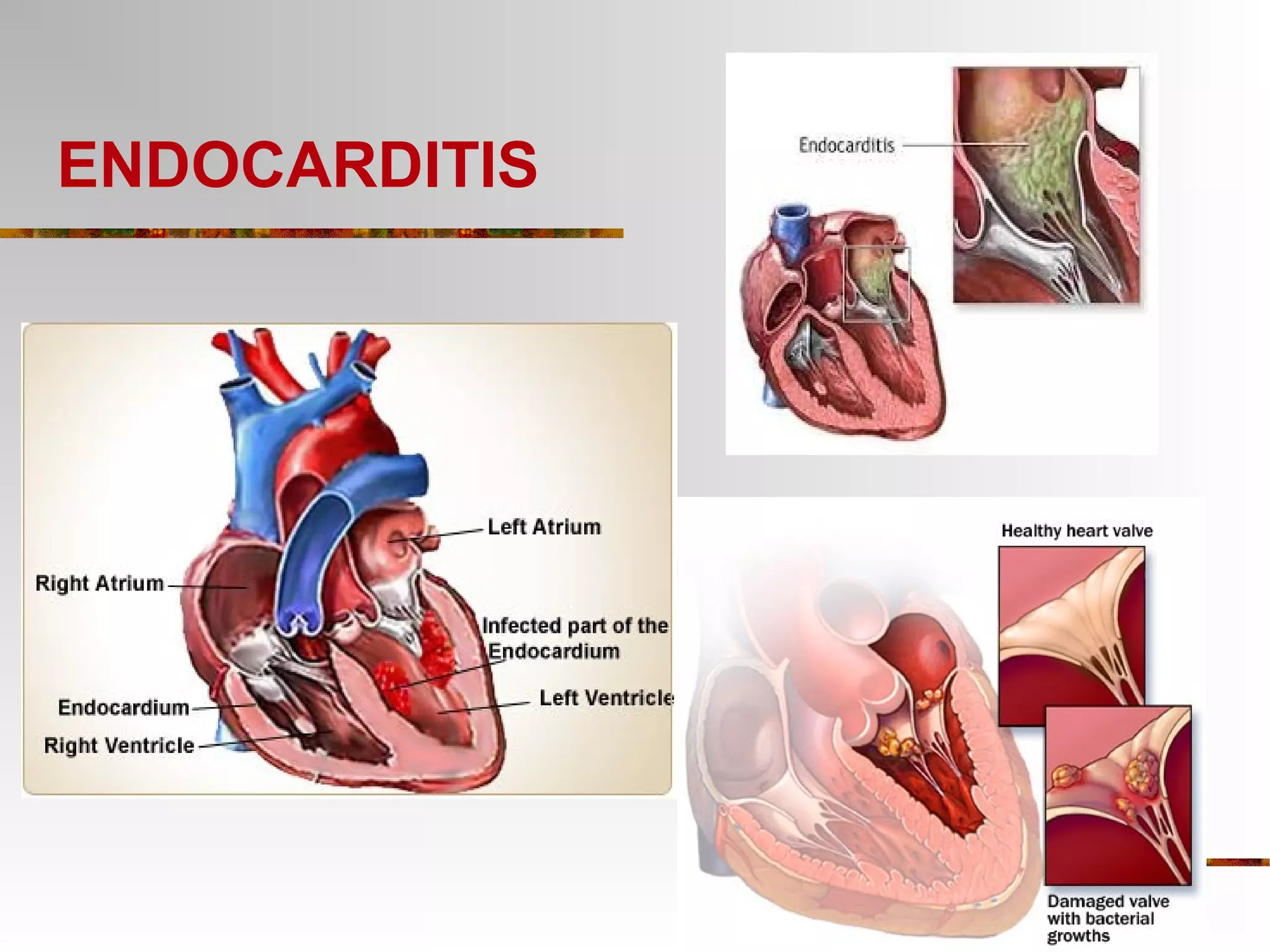
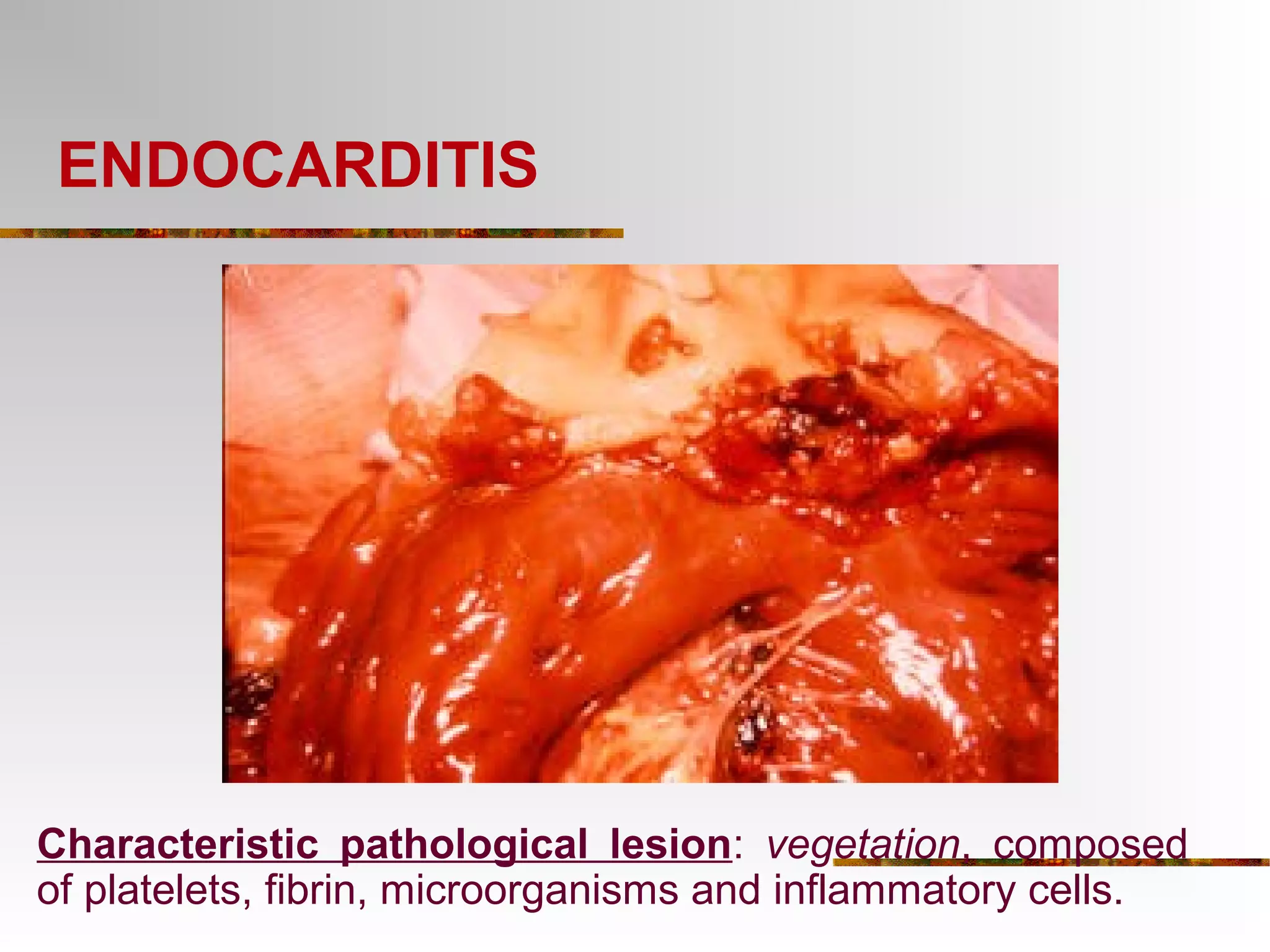
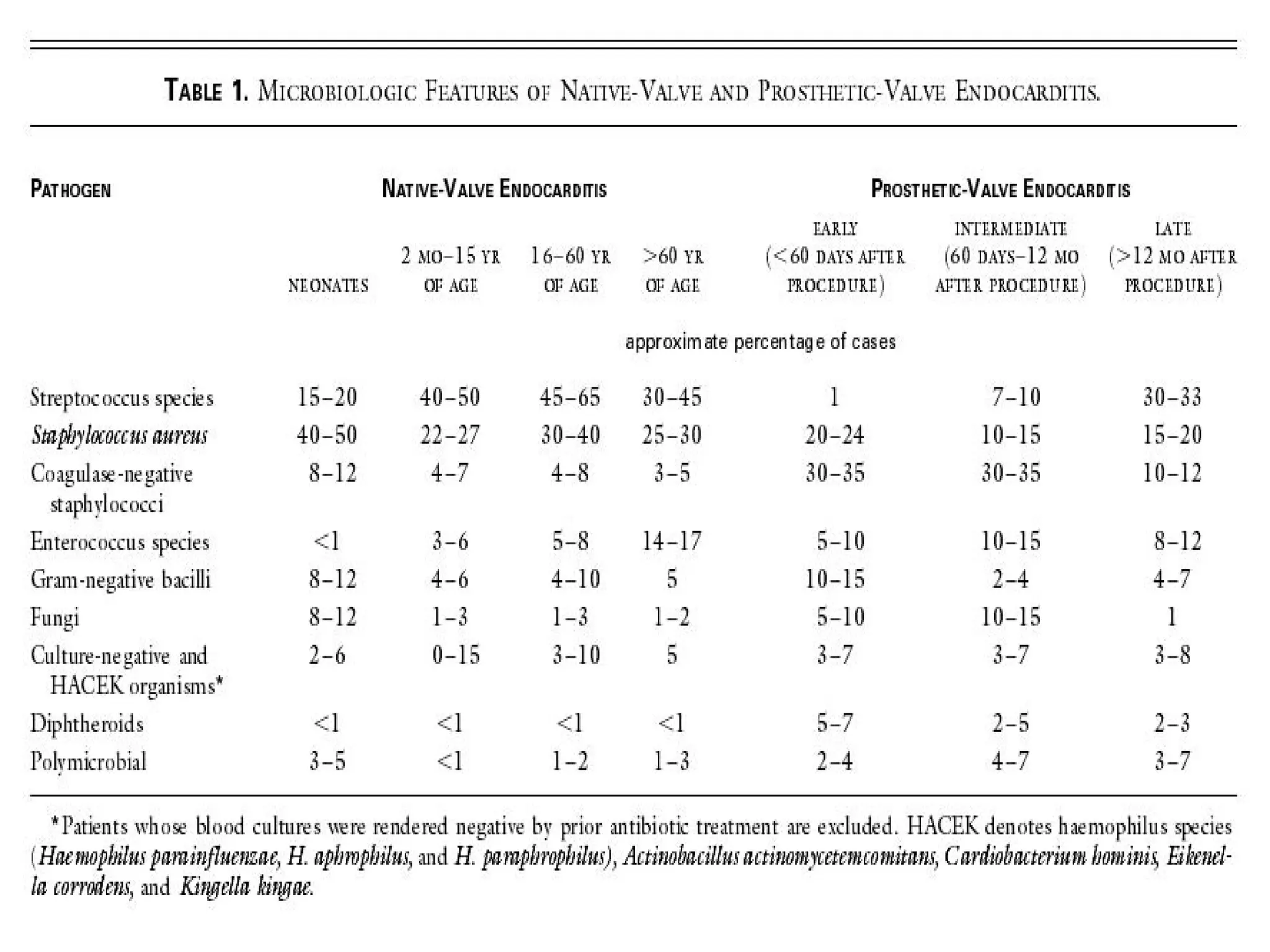
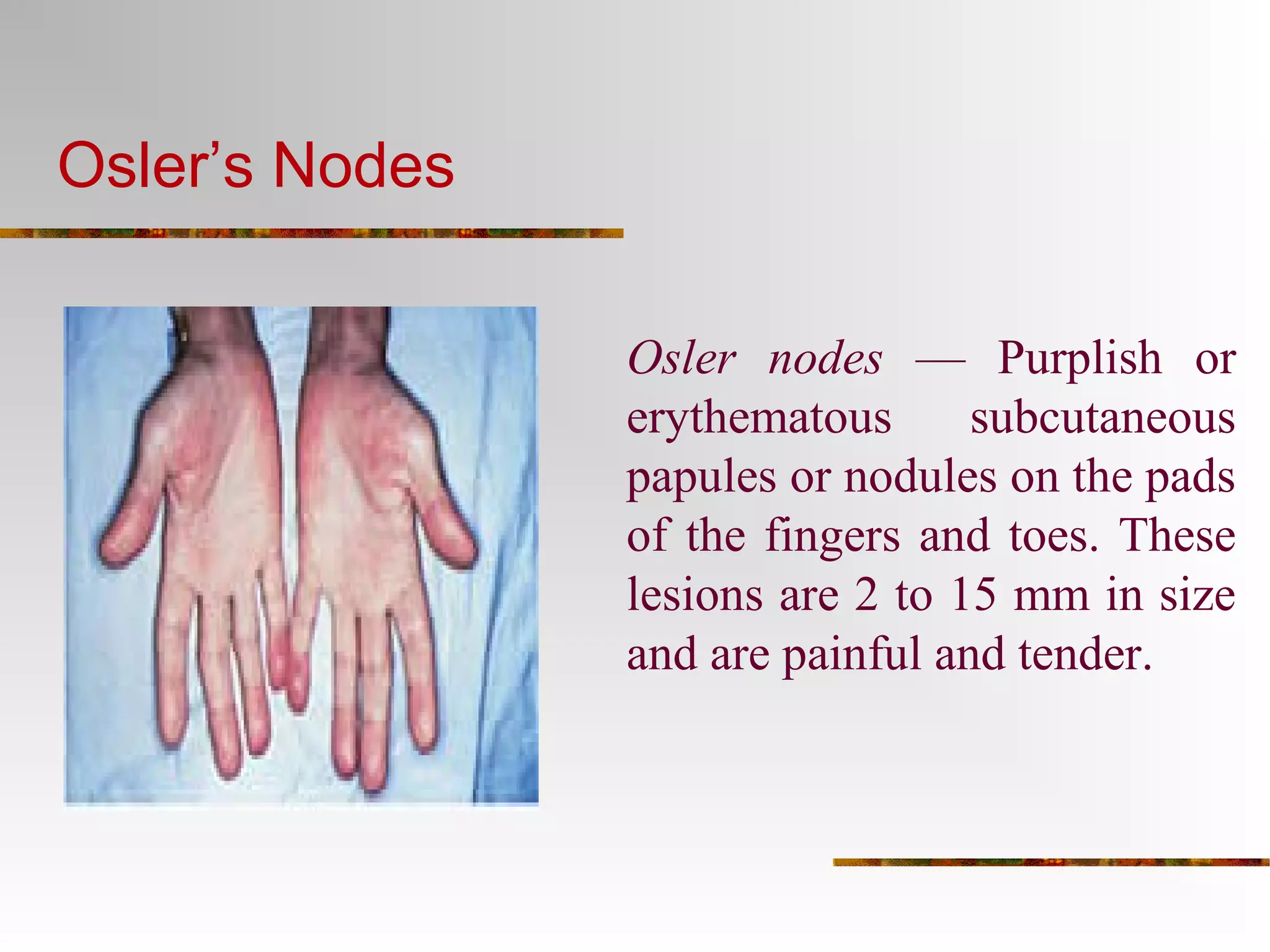
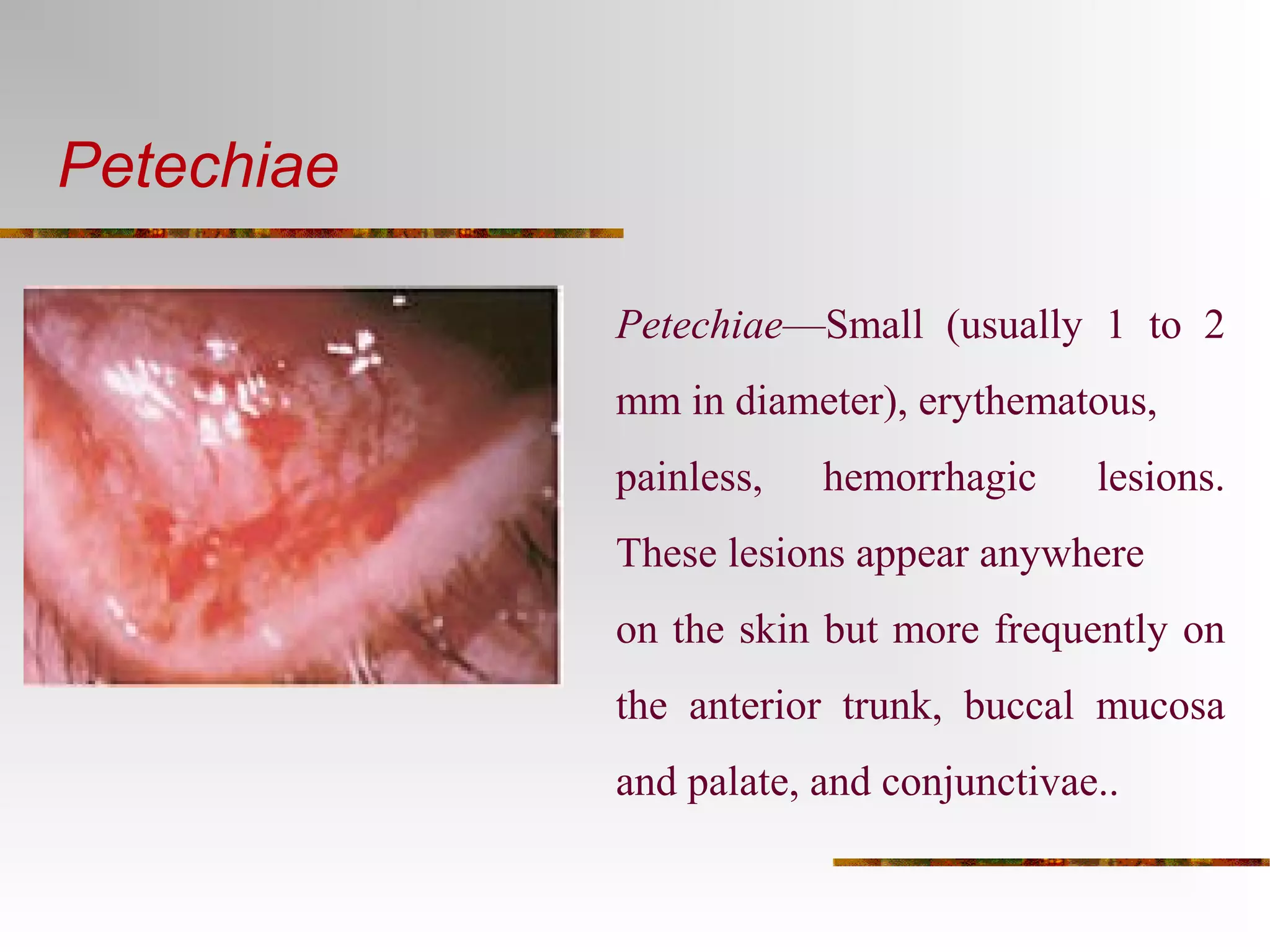
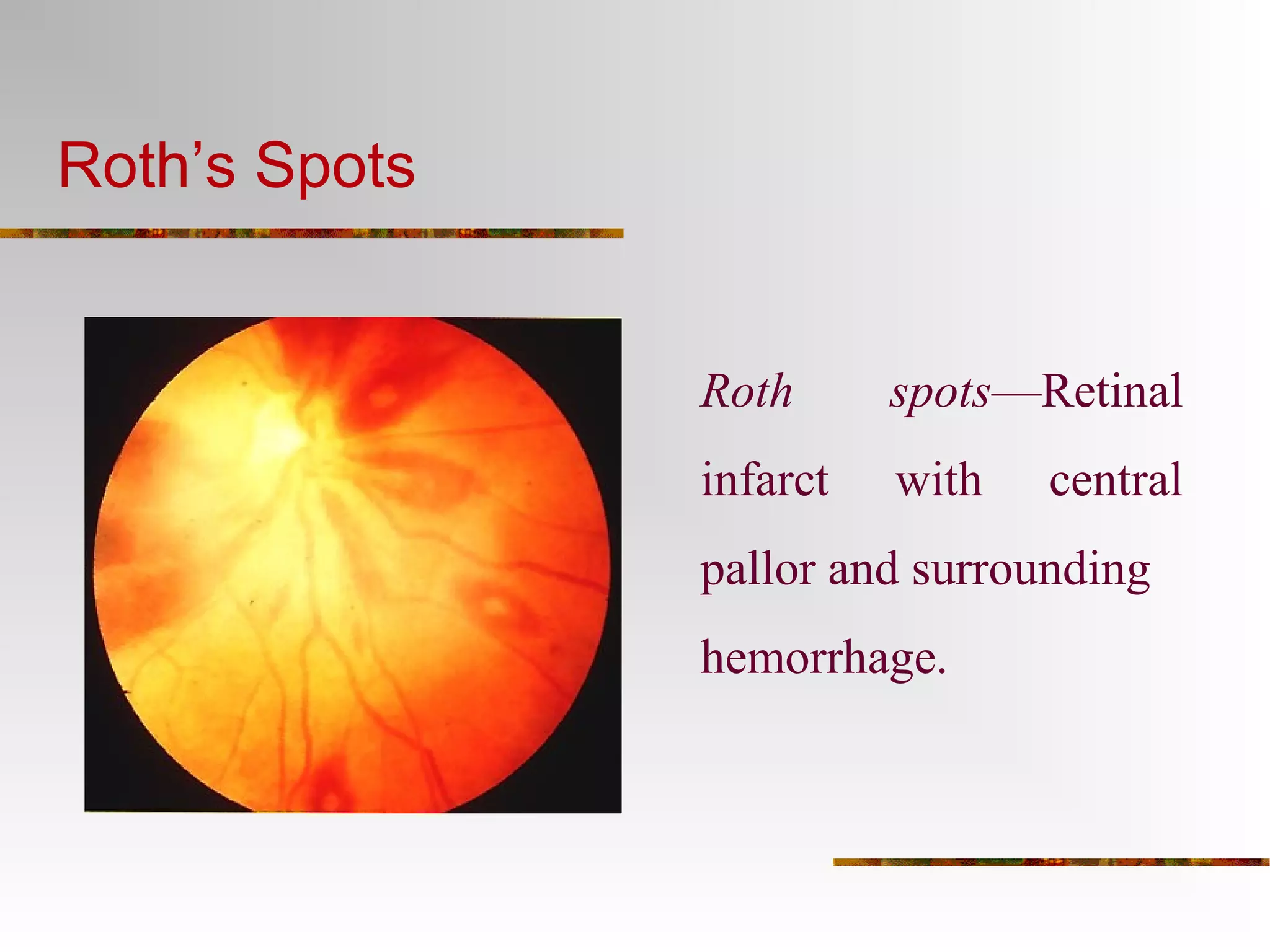
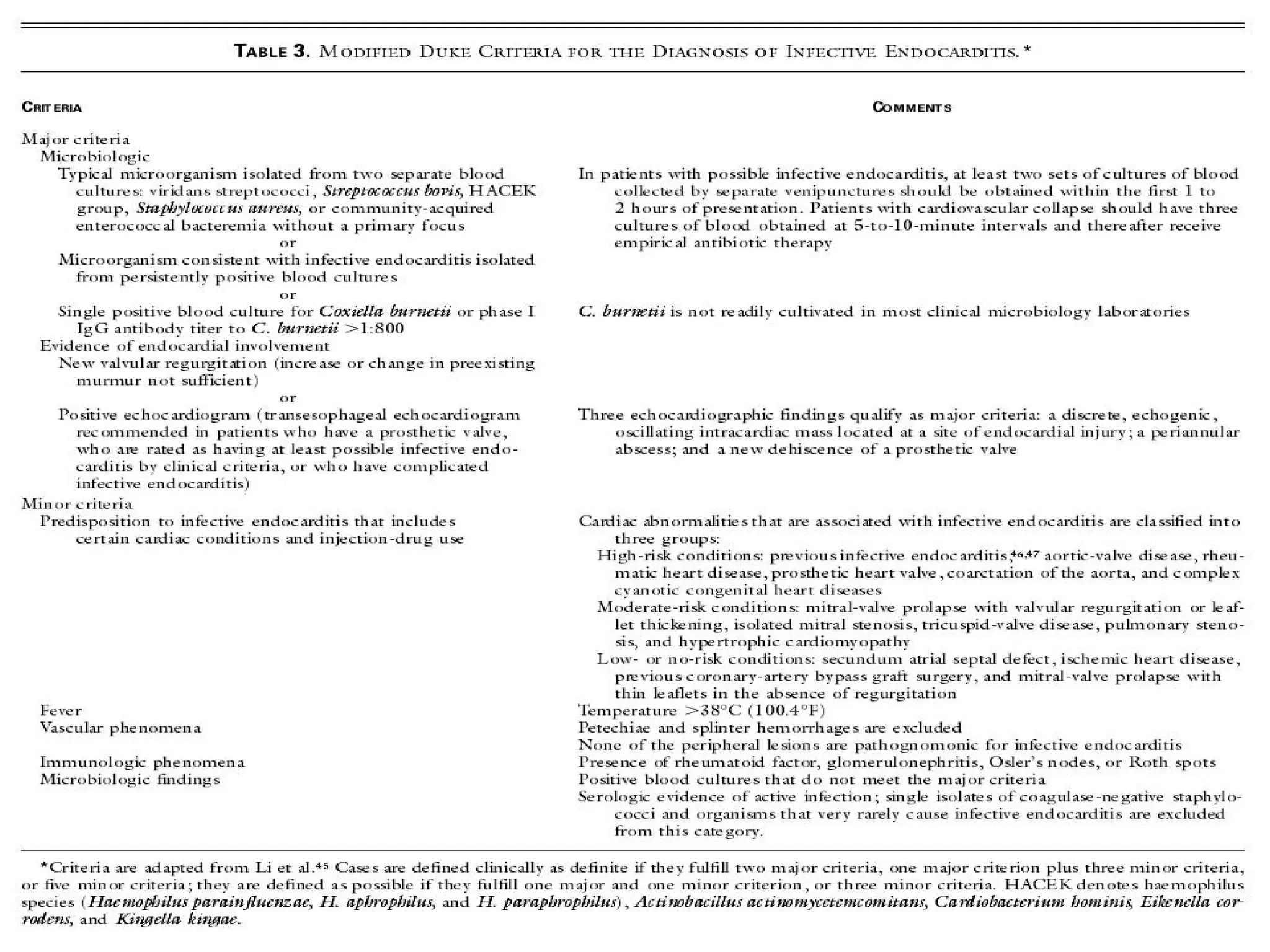
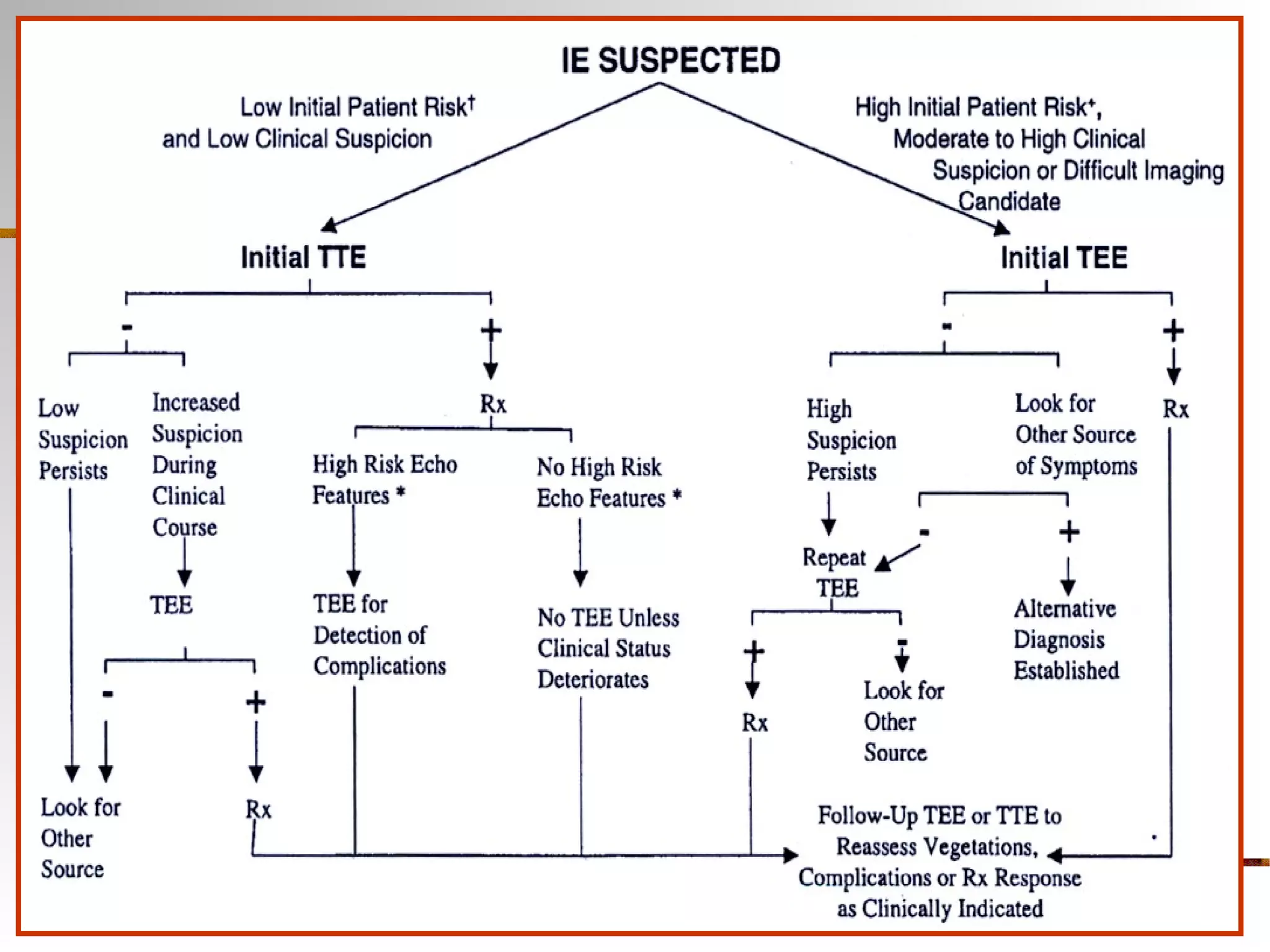
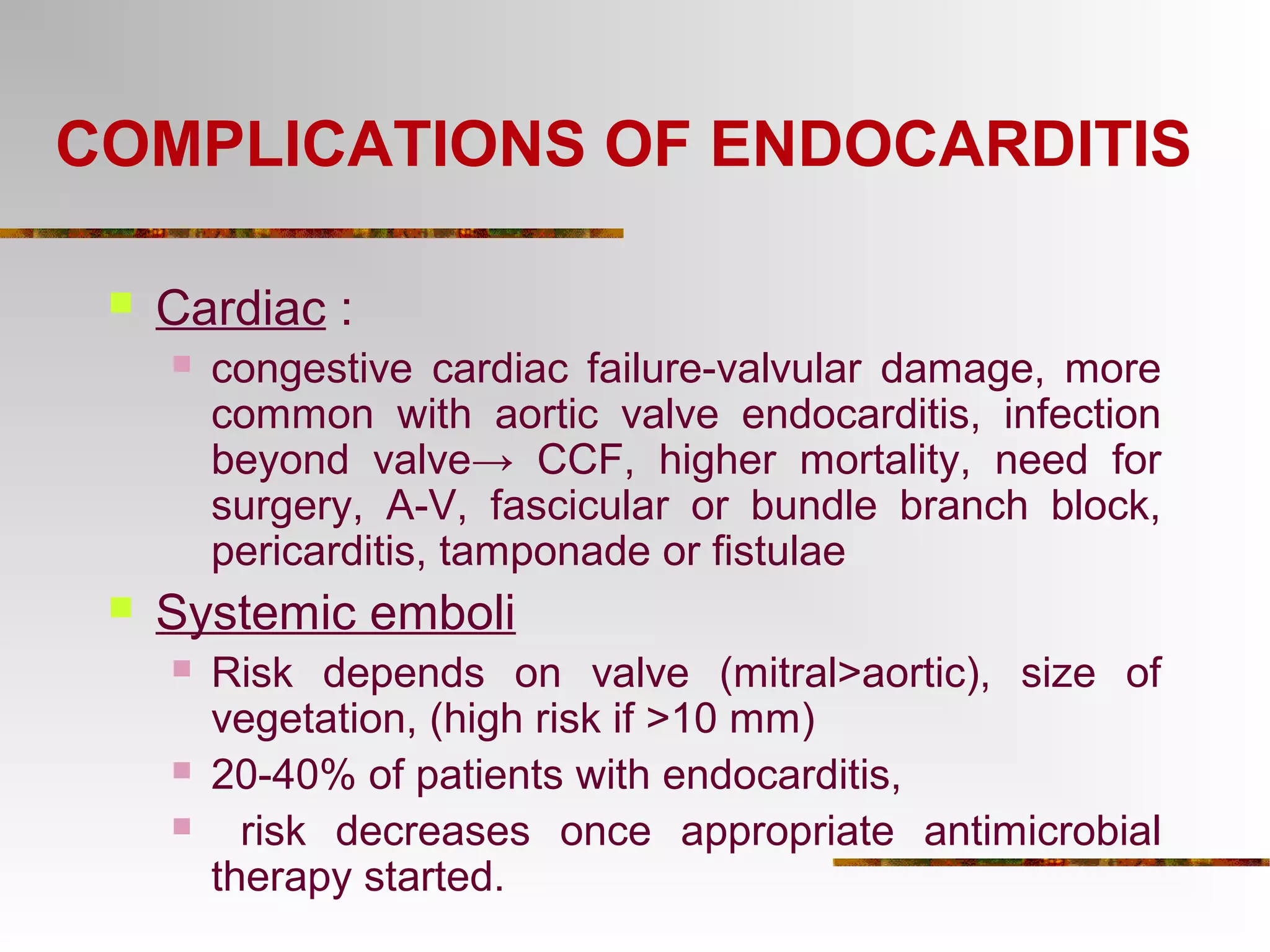
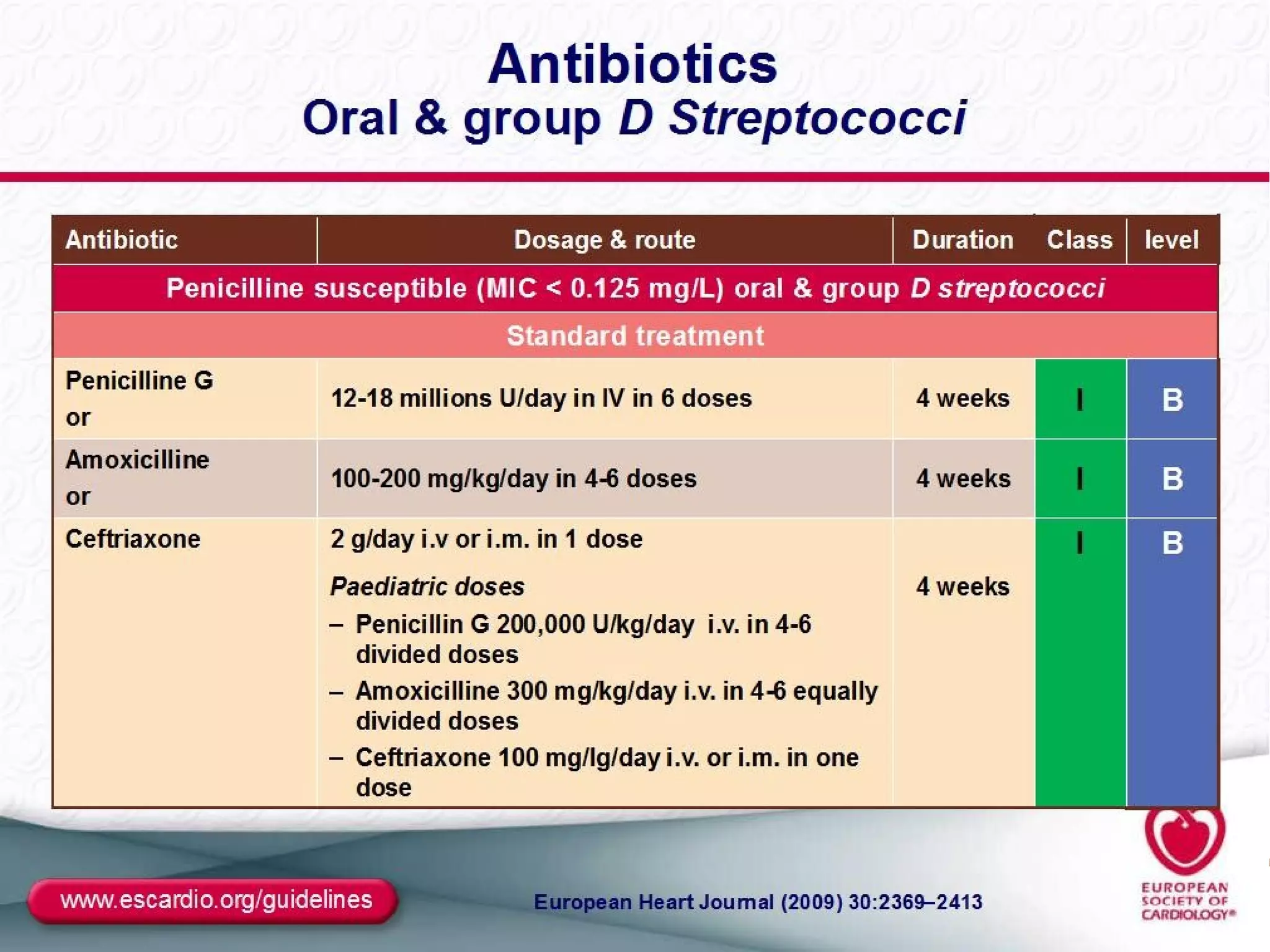
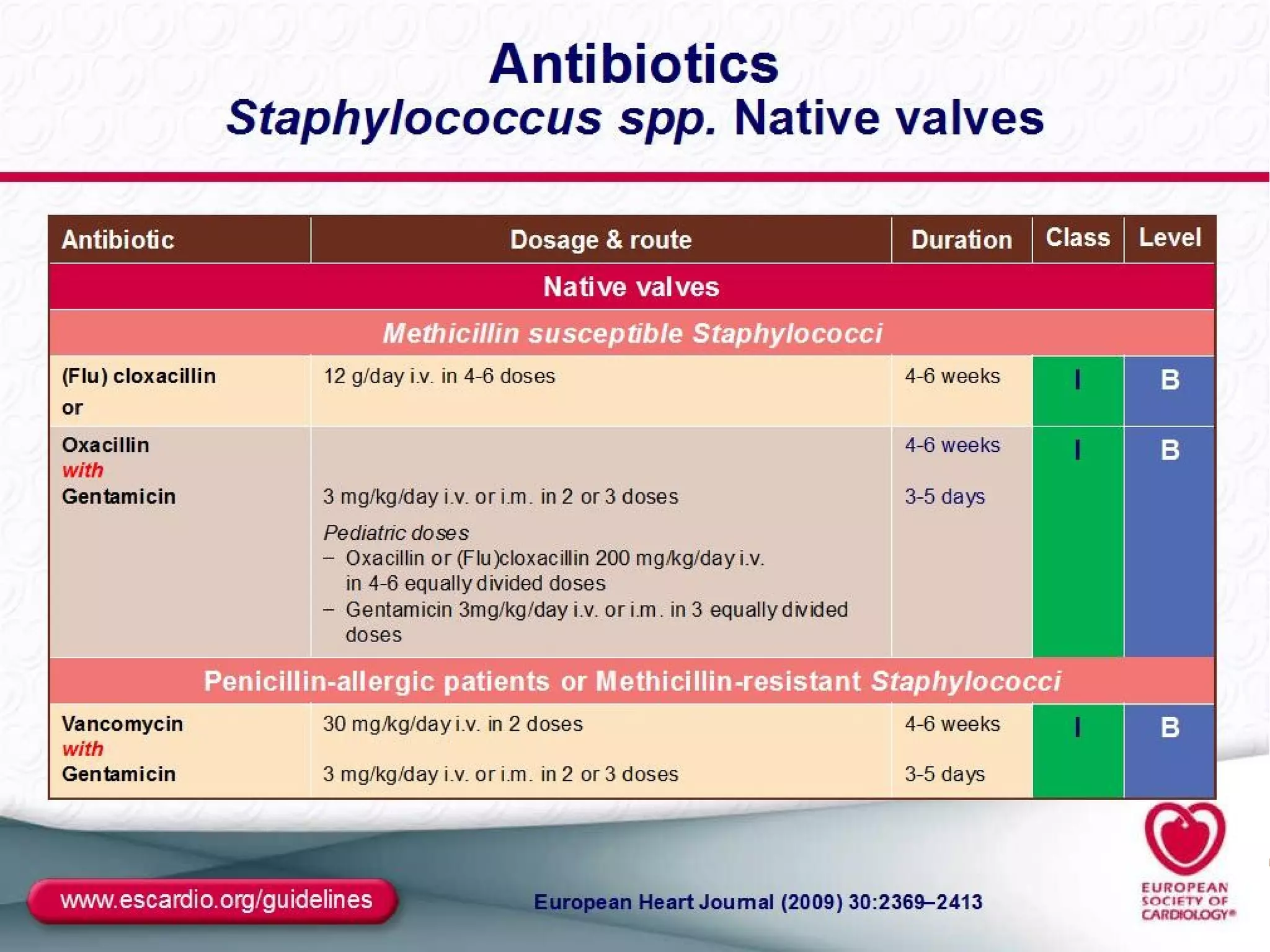
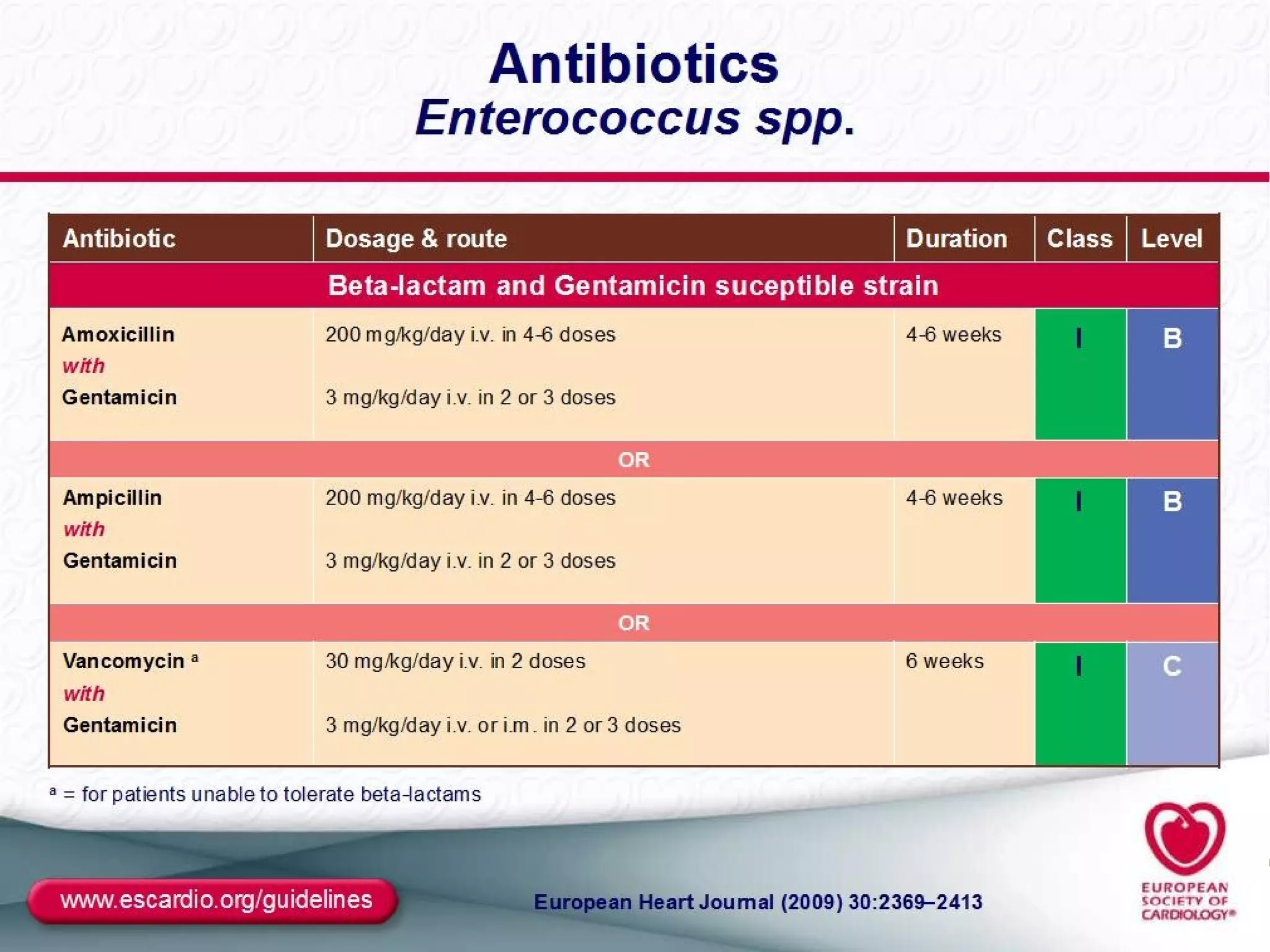
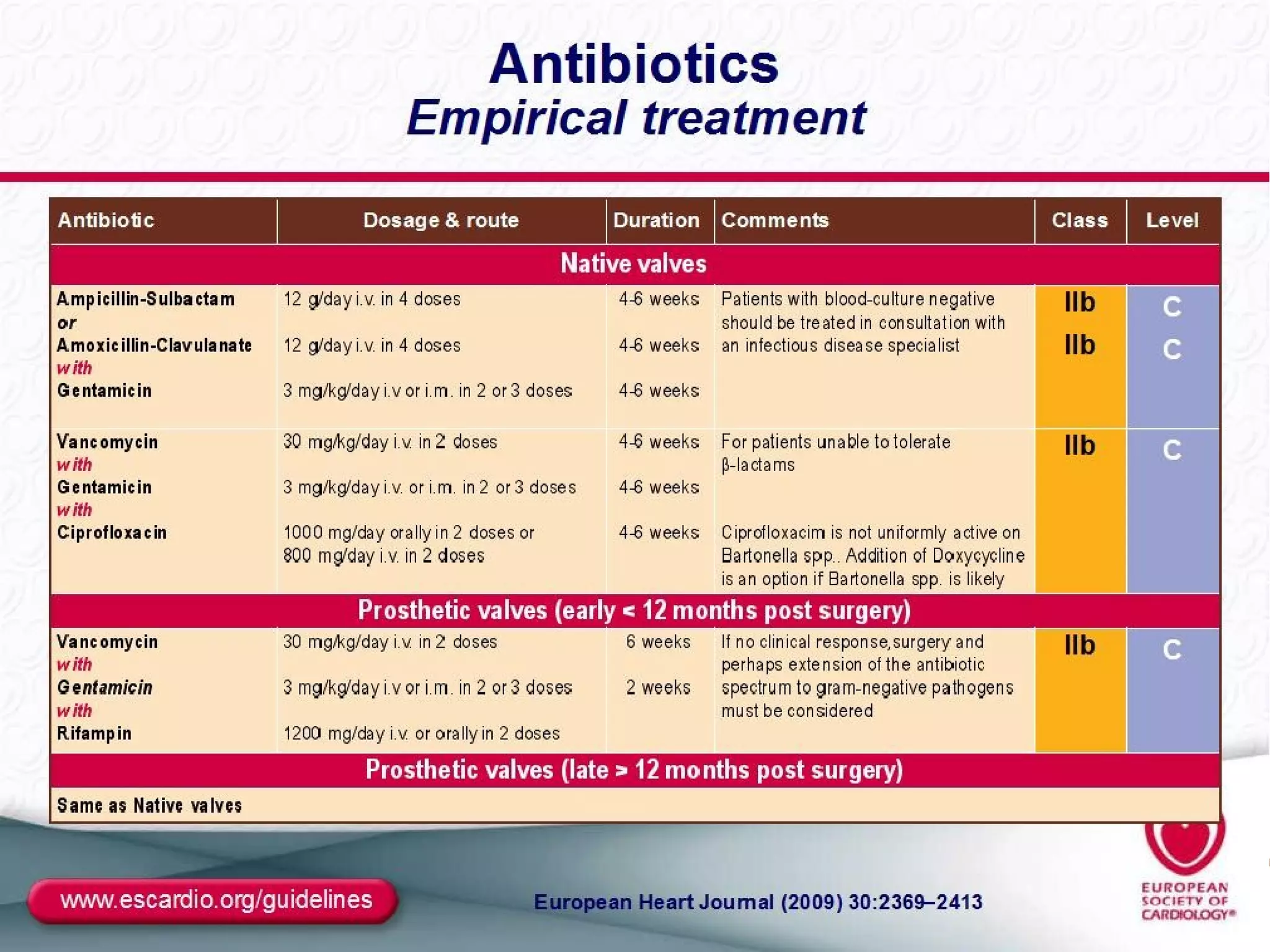
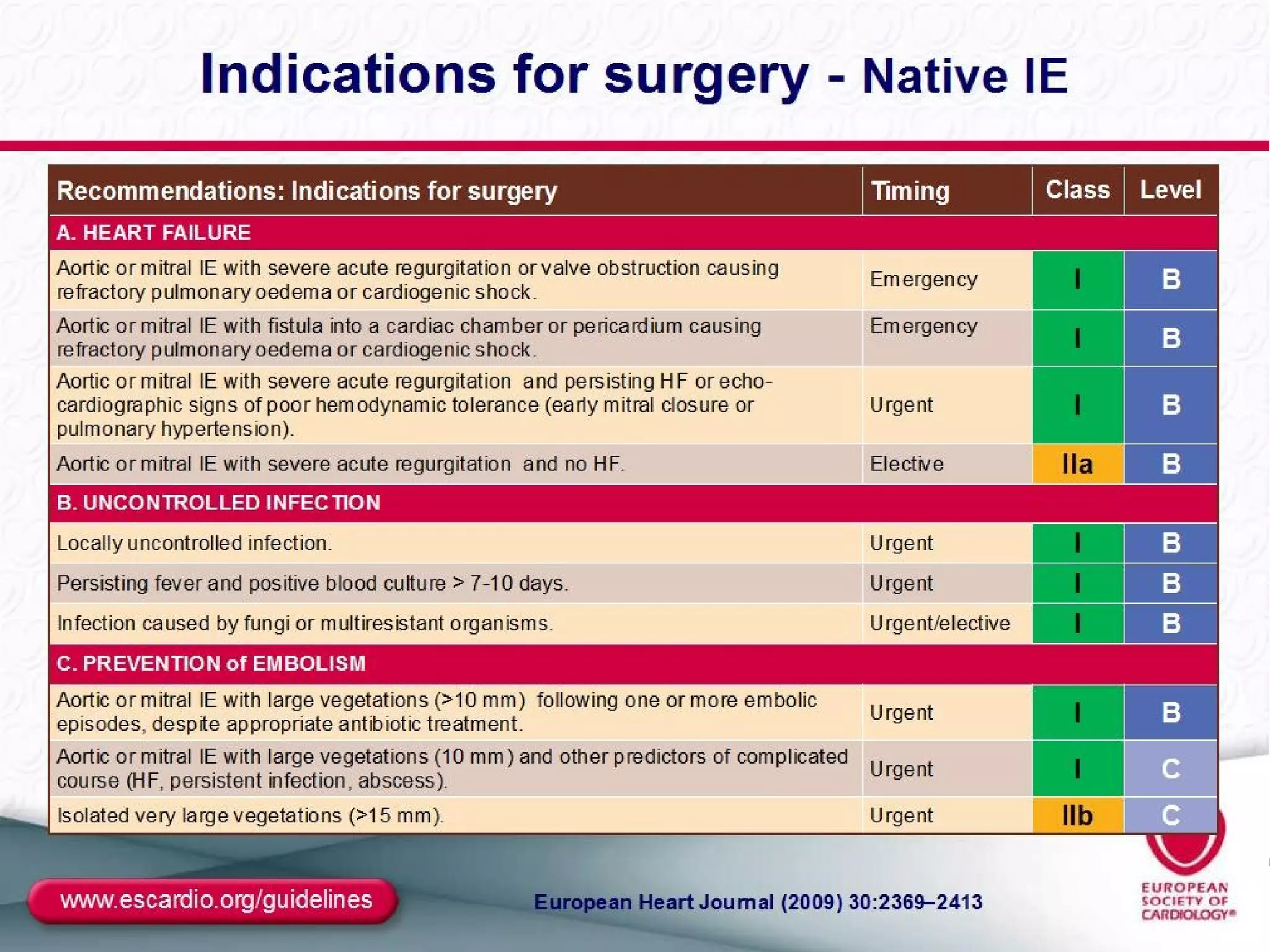
The document provides an extensive overview of infective endocarditis, detailing its definition, epidemiology, clinical presentations, diagnosis, and complications. It highlights risk factors, diagnostic criteria, and the variety of microorganisms that can cause the infection, along with treatment options including antimicrobial therapy and surgical interventions. The Duke criteria for diagnosis and key pathological features associated with the disease are also emphasized.