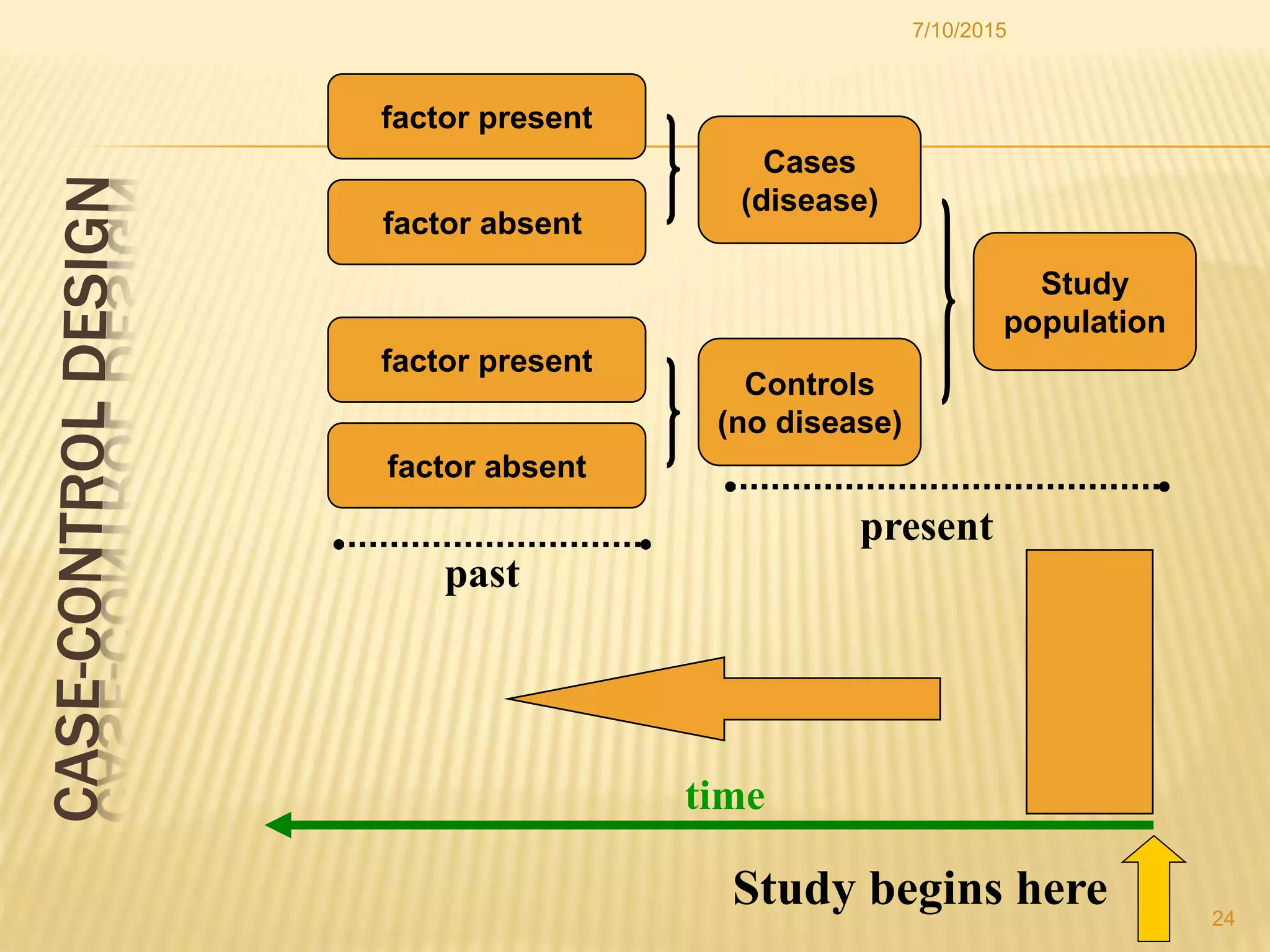
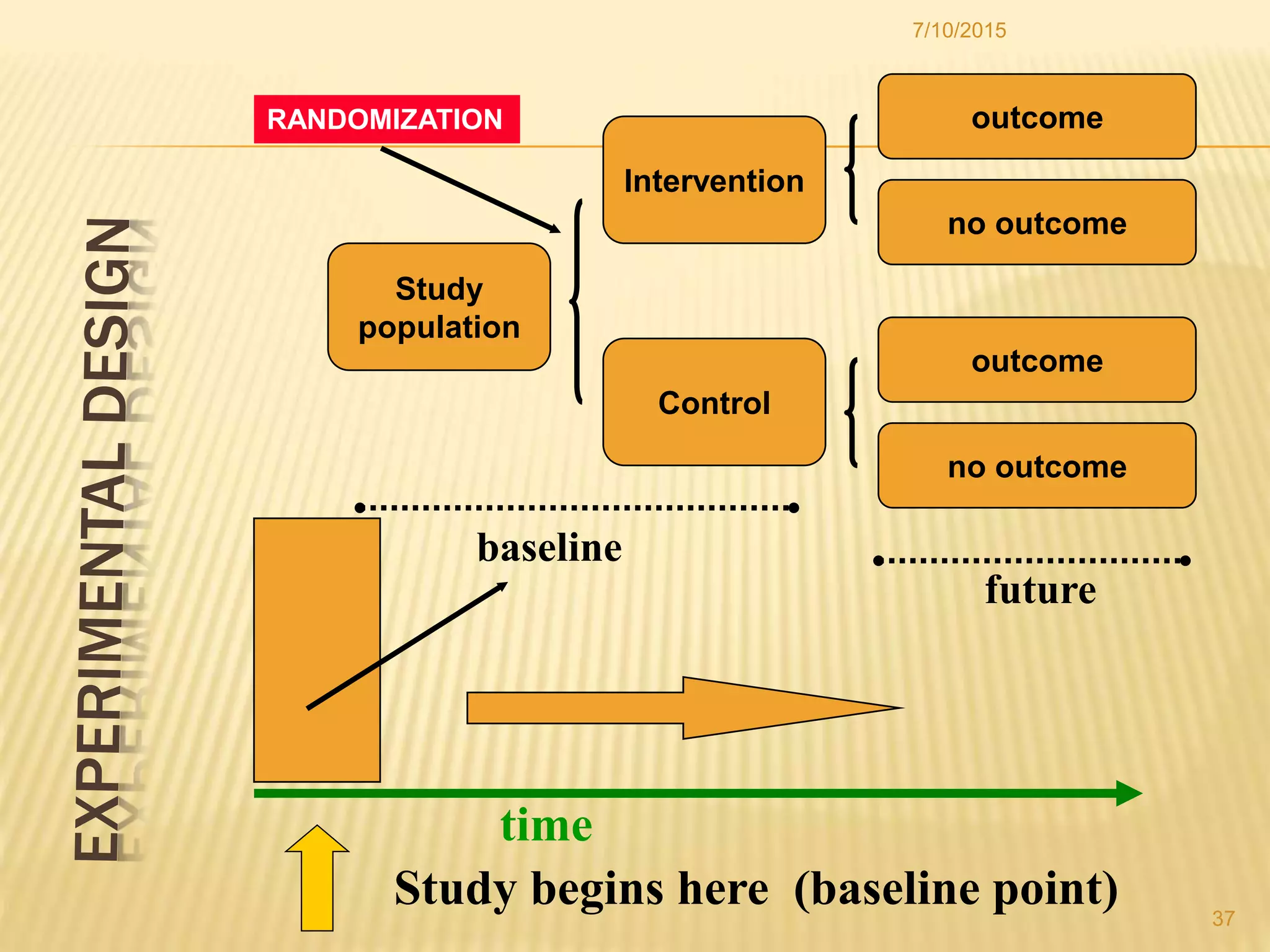
The document discusses different study designs used in research, including observational studies like case reports, case series, cross-sectional studies, and cohort studies, as well as experimental studies like randomized controlled trials. It describes the key characteristics and advantages and disadvantages of each design. The highest level of evidence comes from randomized controlled trials, while observational studies are useful for initial hypothesis generation.