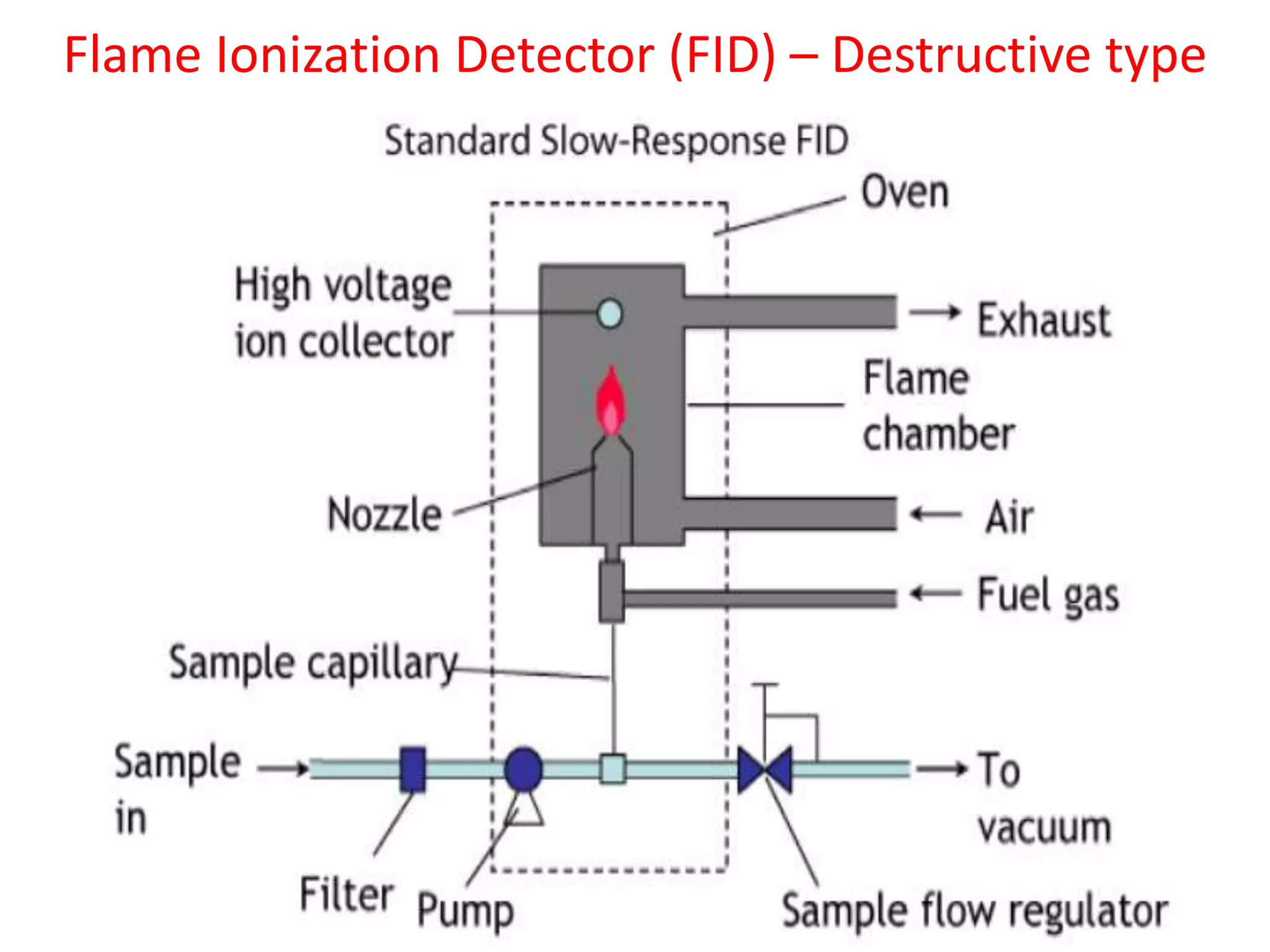
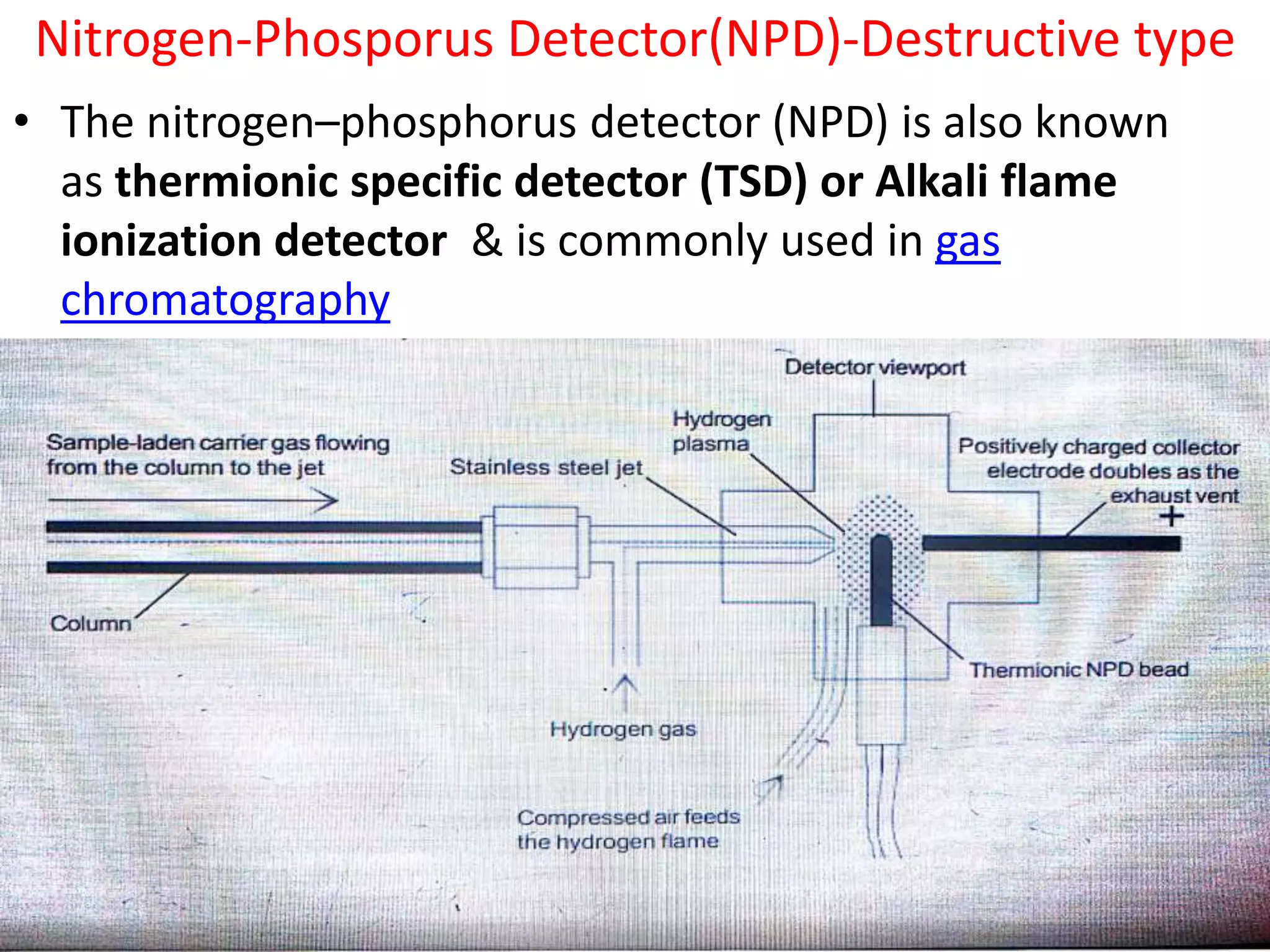
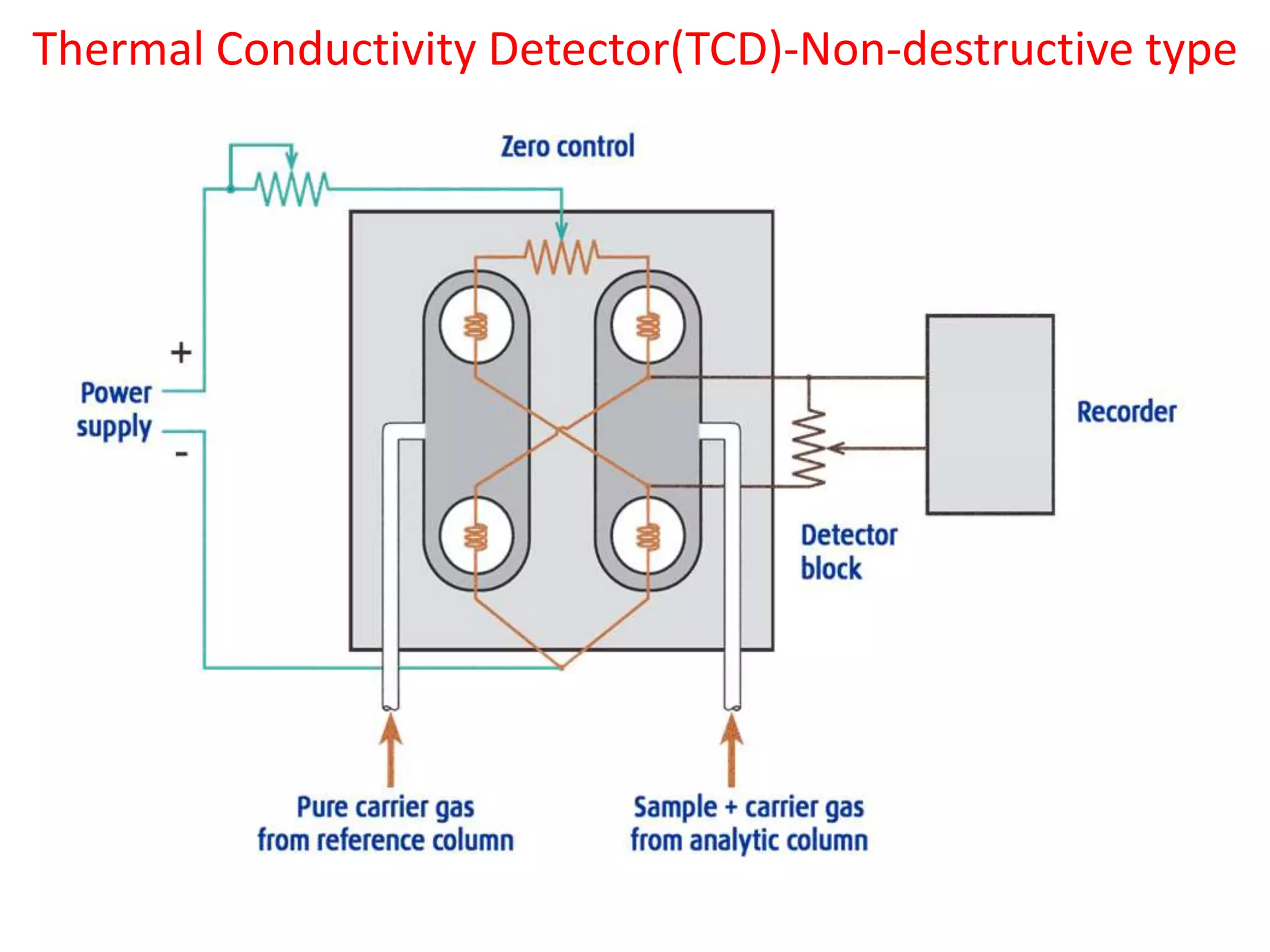
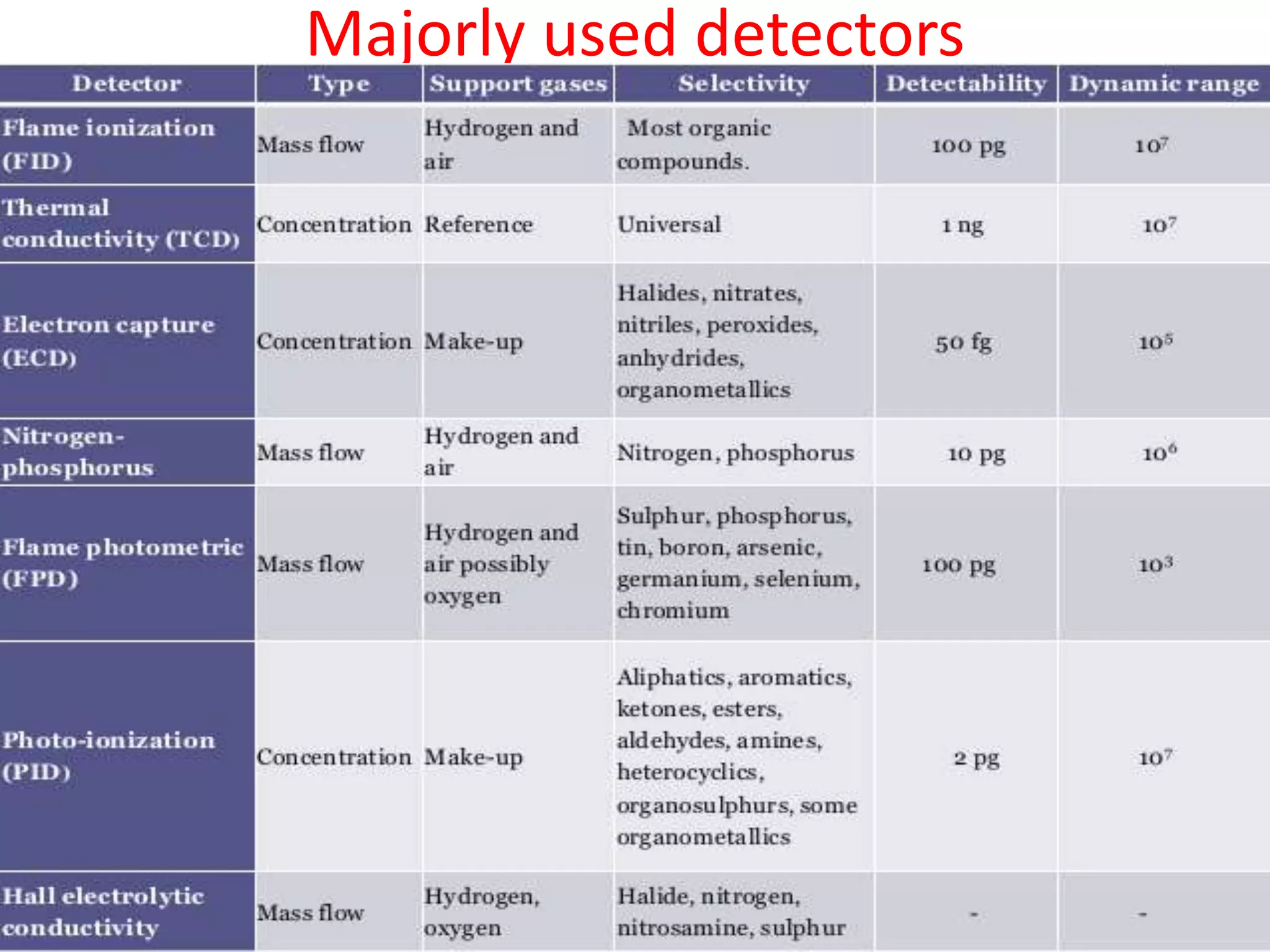
Detectors are devices used in gas chromatography and liquid chromatography to detect components of mixtures being analyzed. There are two main types of detectors: destructive and non-destructive. Destructive detectors transform the analyte through burning, evaporation, or mixing with reagents before measurement, such as the flame ionization detector (FID) and nitrogen phosphorus detector (NPD). Non-destructive detectors directly measure properties like UV absorption or thermal conductivity without transforming the analyte, exemplified by the thermal conductivity detector. The most commonly used detectors are the FID, NPD, and TCD due to their high sensitivity, reproducibility, and selectivity for certain compounds.