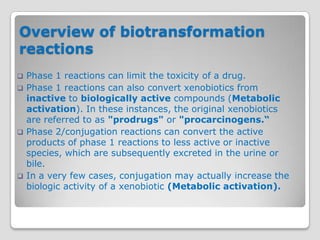
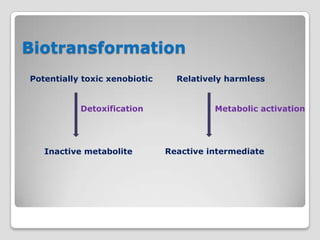
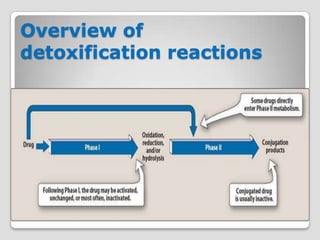
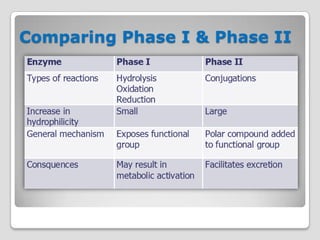
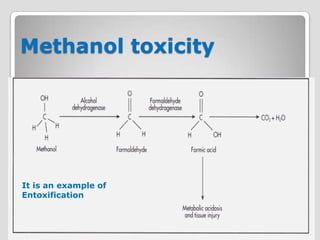
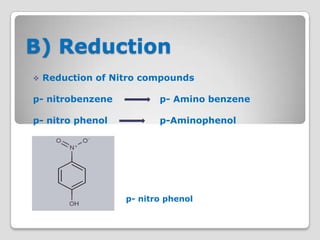
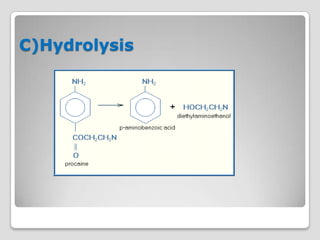
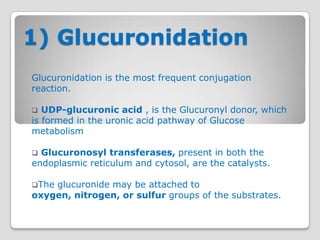
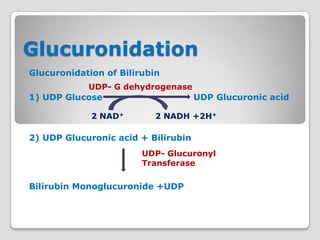
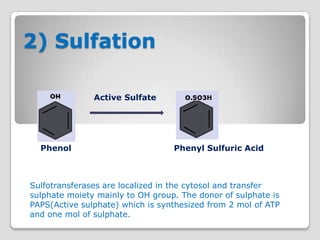
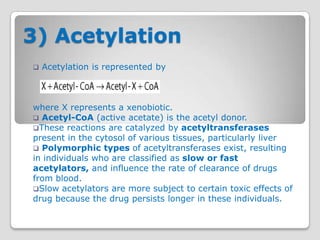
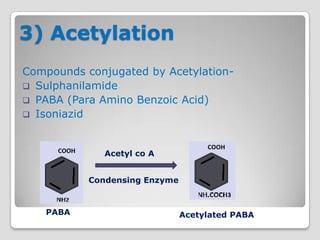
The document discusses biotransformation and detoxification reactions. It describes how xenobiotics are metabolized in two phases: Phase 1 involves reactions like hydroxylation and Phase 2 involves conjugating these products to make them more hydrophilic and excretable, through glucuronidation, sulfation, acetylation, methylation or conjugation to amino acids or glutathione. The cytochrome P450 system is important for Phase 1 reactions like oxidation. Phase 2 makes compounds more polar through conjugating them to compounds like glucuronic acid. This allows xenobiotics to be safely eliminated from the body.





































![Effects of Xenobiotics
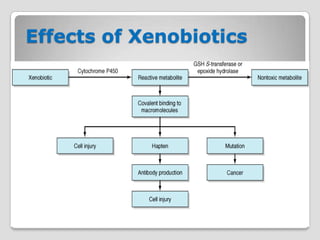
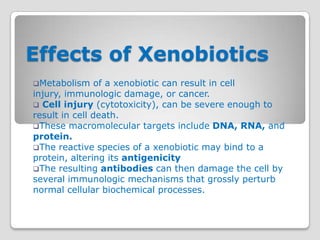
Reactions of activated species of chemical carcinogens
with DNA are of great importance in chemical
carcinogenesis
Some chemicals (eg, benzo[α]pyrene) require
activation by monooxygenases in the endoplasmic
reticulum to become carcinogenic (they are thus called
indirect carcinogens).
The products of the action of certain monooxygenases
on some procarcinogen substrates are epoxides.
Epoxides are highly reactive and mutagenic or
carcinogenic or both.
Epoxide hydrolase—like cytochrome P450acts on these
compounds, converting them into much less reactive
dihydrodiols.](https://image.slidesharecdn.com/xenobiotics-121123112739-phpapp02/85/Xenobiotics-53-320.jpg)
