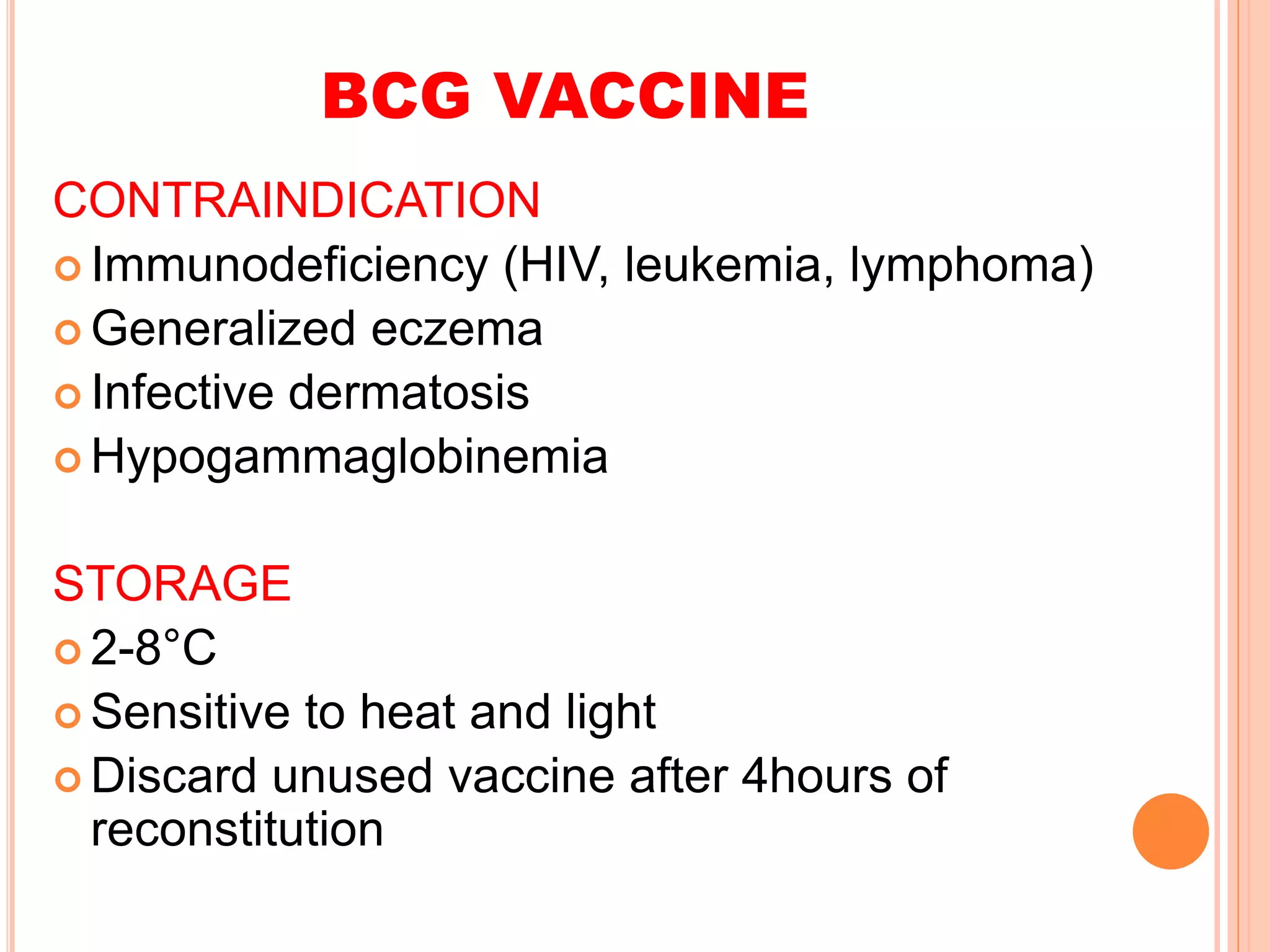
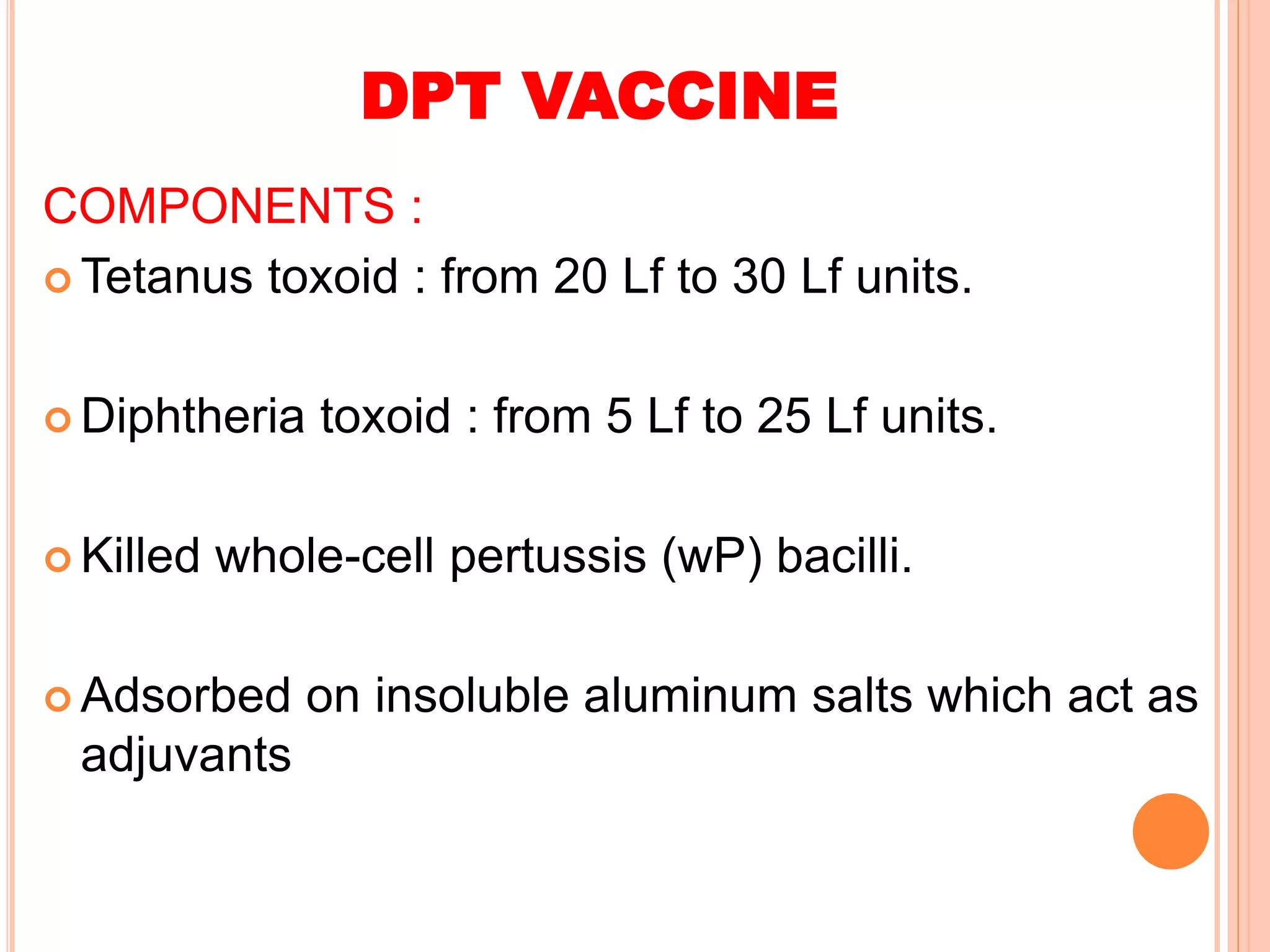
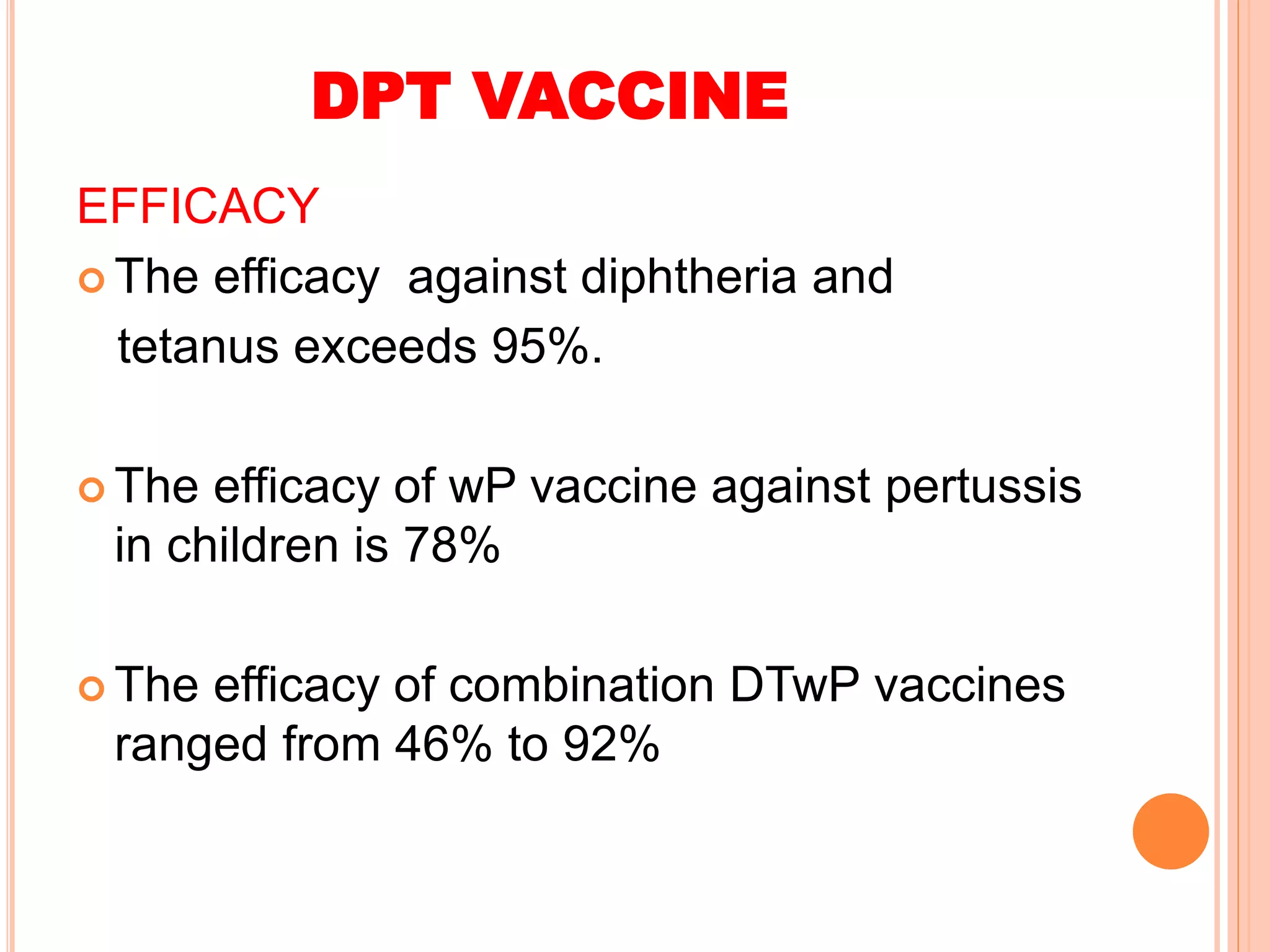
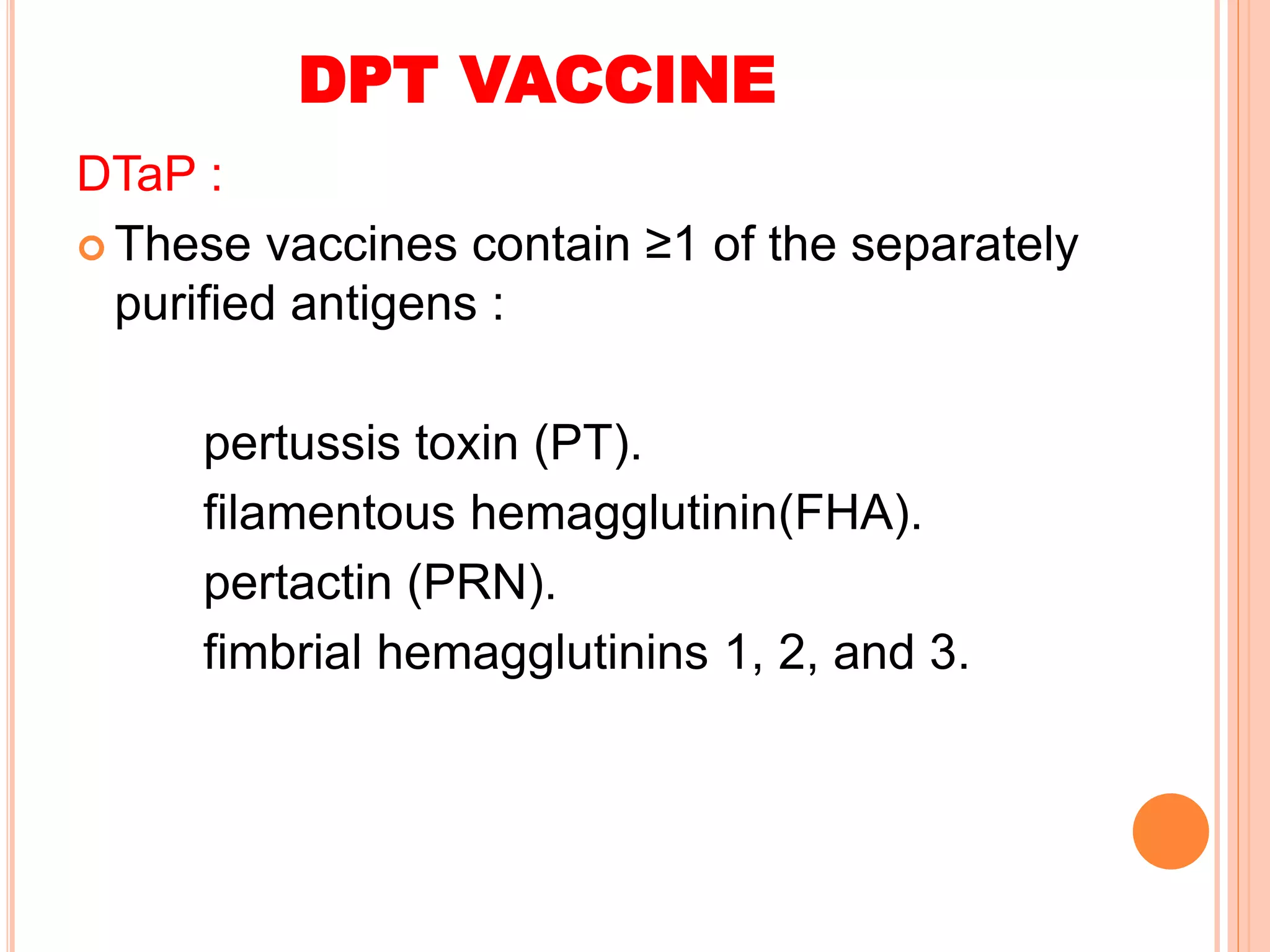
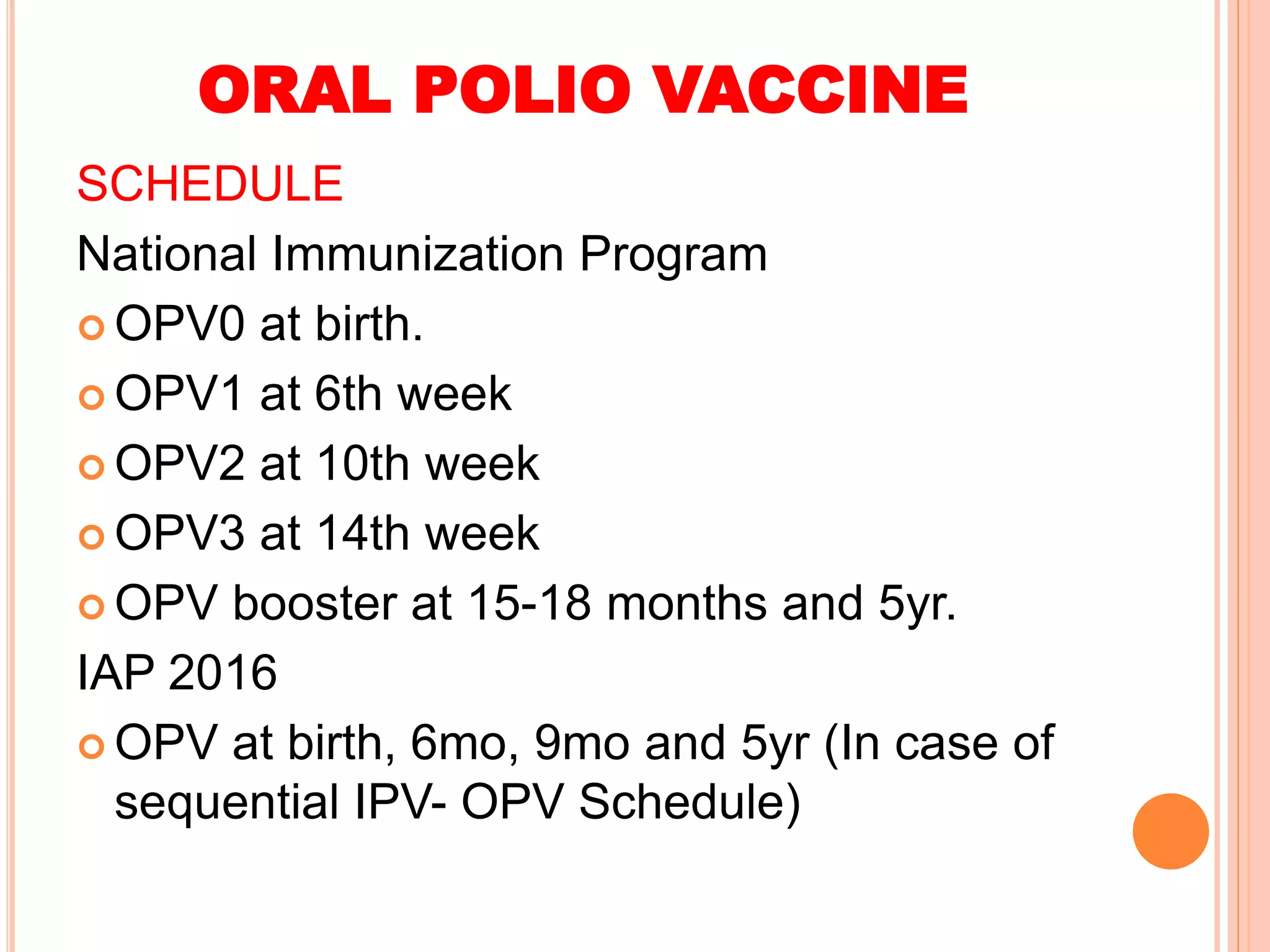
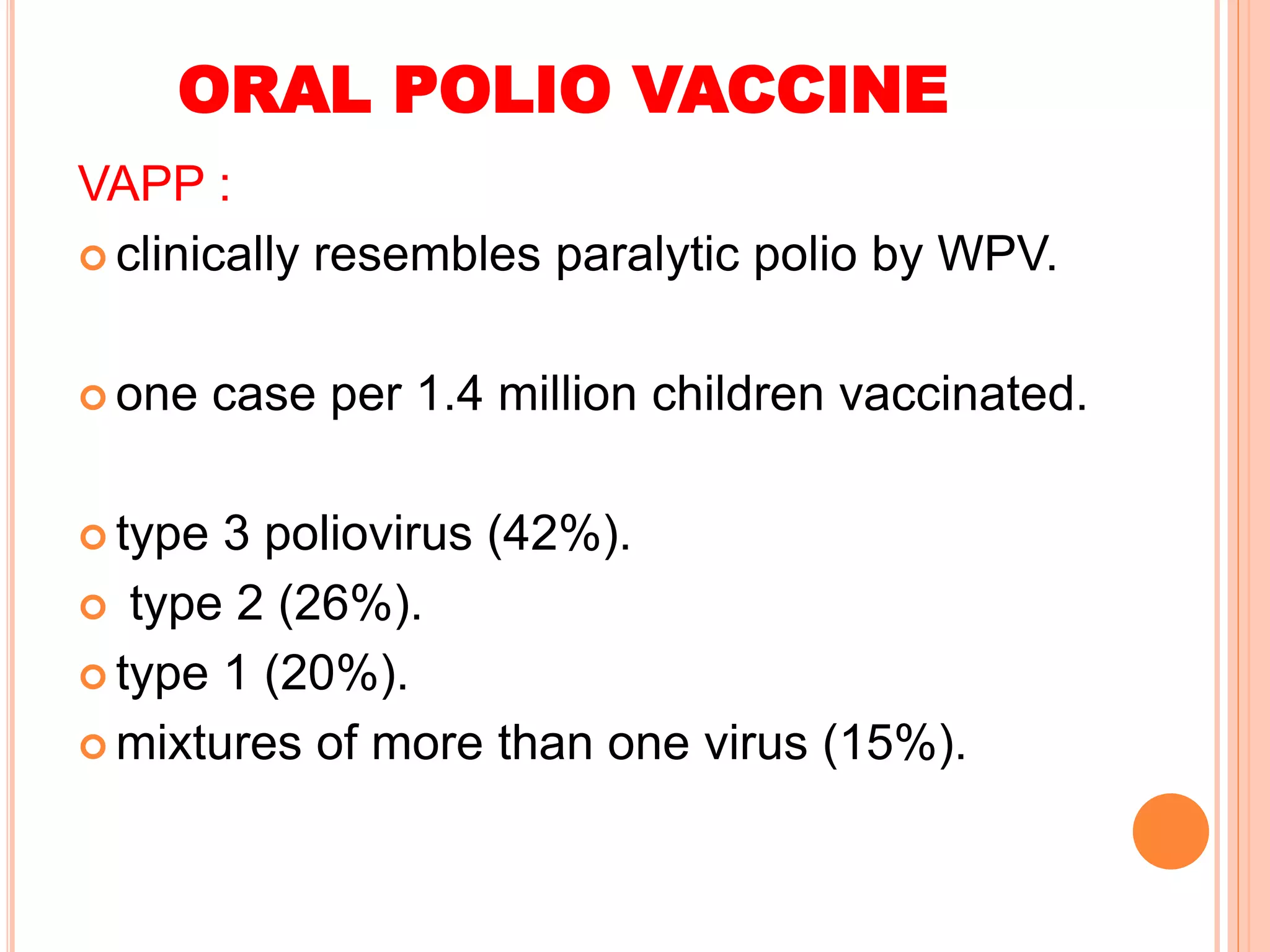
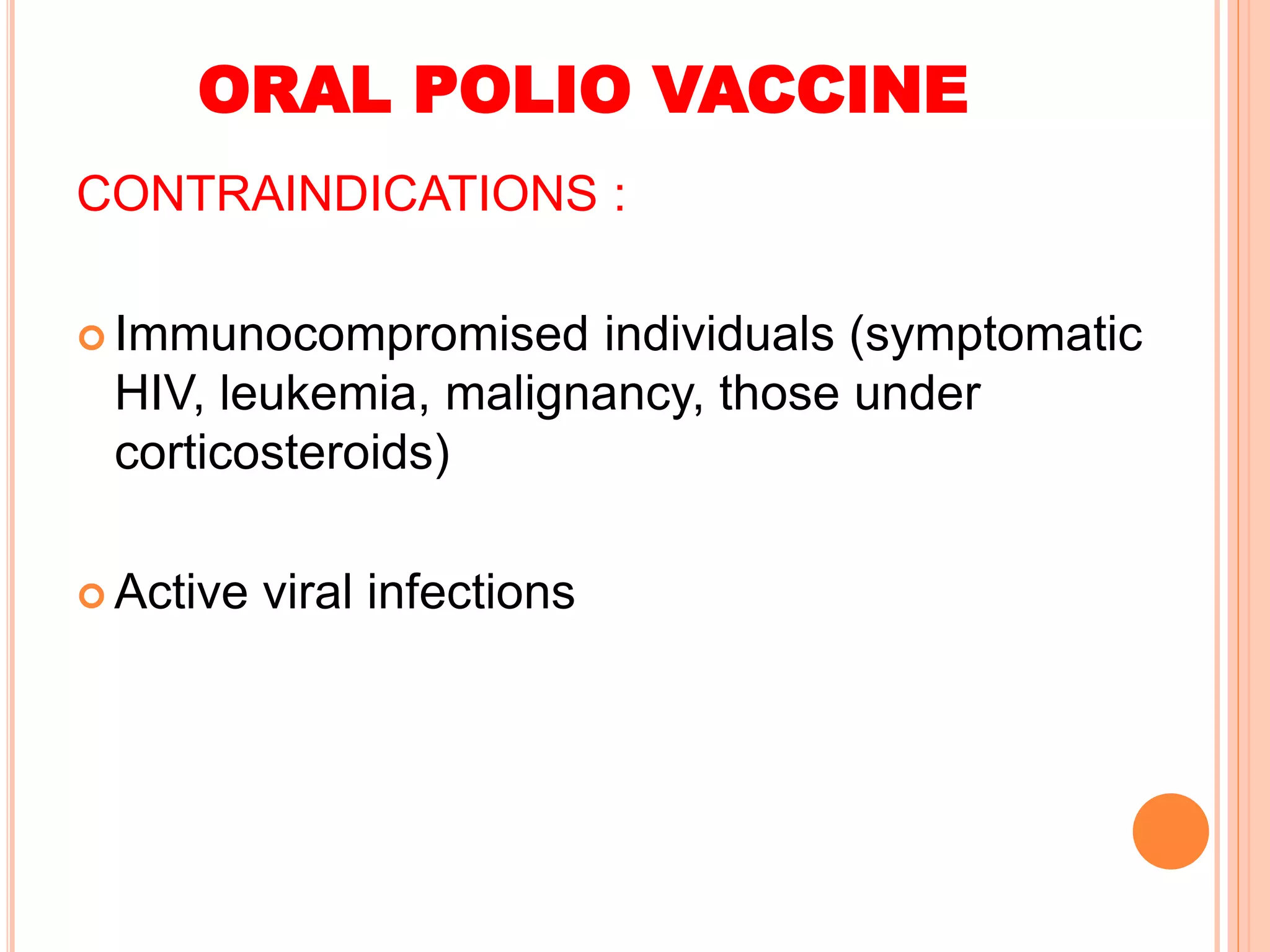
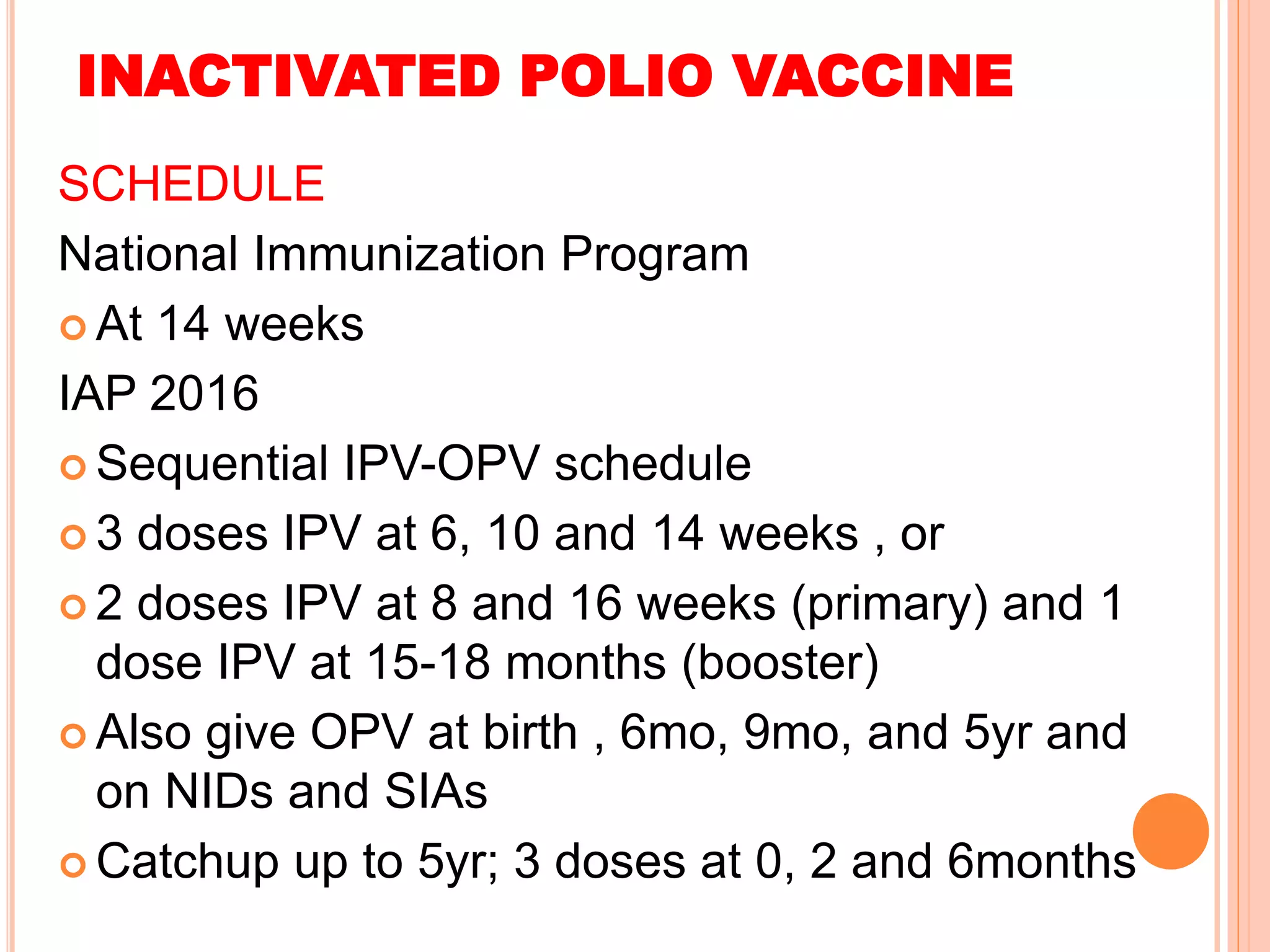
The document summarizes information about various vaccines including BCG, DPT, oral polio vaccine, and inactivated polio vaccine. It discusses details like the epidemiology of the diseases they protect against, how the vaccines are produced and administered, their efficacy and safety, recommended schedules, storage requirements, and contraindications. The vaccines protect against tuberculosis, diphtheria, pertussis, tetanus, and poliomyelitis, and reducing the burden of these diseases has been a priority for global immunization programs.