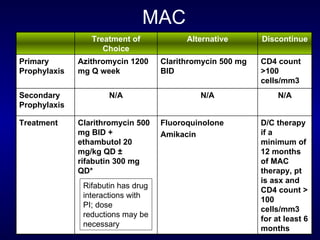
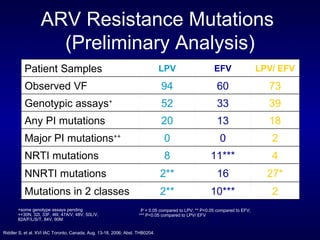
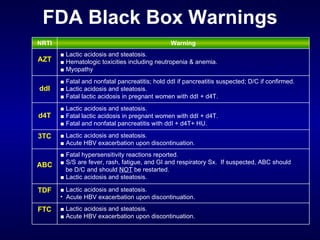
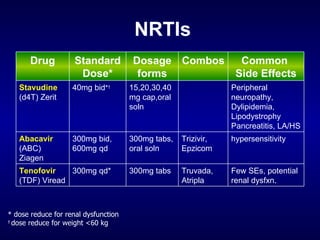
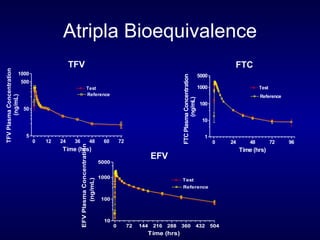
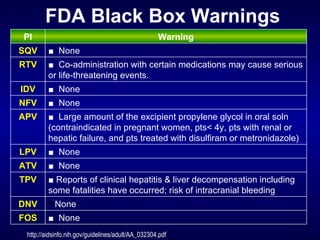
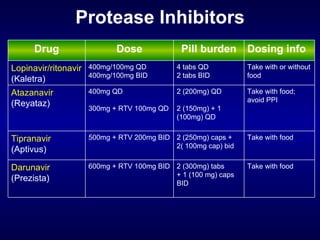
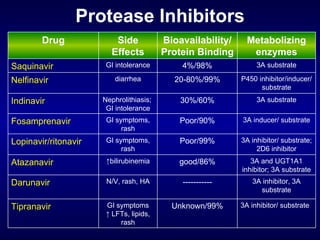
The document provides an update on HIV treatment guidelines for treatment-naïve patients. It discusses the goals of antiretroviral therapy, current treatment recommendations including preferred combination regimens, and results from clinical trials comparing different regimen options. New data on TDF/FTC/EFV versus CBV/EFV and LPV/r-based versus EFV-based regimens are presented, showing benefits to initial treatment with certain preferred regimens.


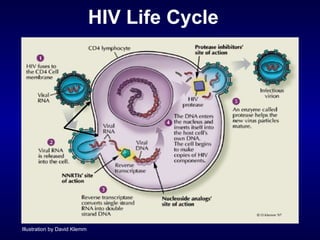



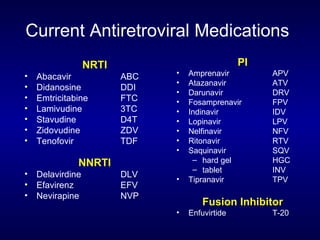
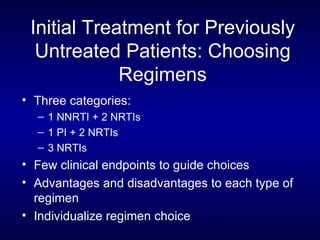

































![Entry Inhibitors Fusion inhibitors [Fuzeon (enfuvirtide, T20)] Attachment Inhibitors Chemokine co-receptor antagonists See Kilby and Eron, NEJM 2003;348:2228-38](https://image.slidesharecdn.com/1822498/85/11-49-320.jpg)