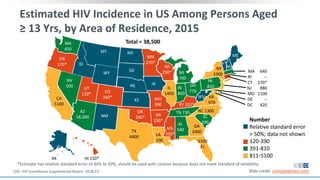
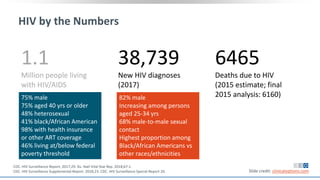
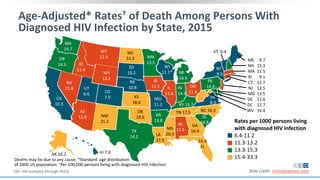
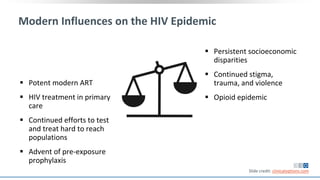
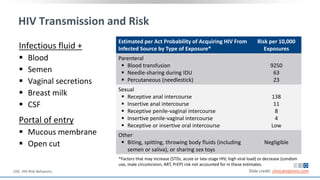
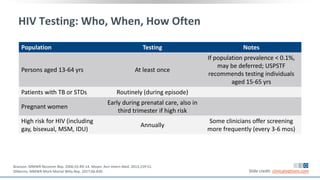
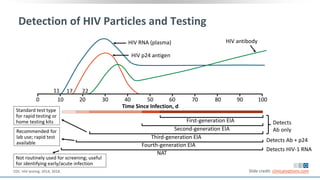
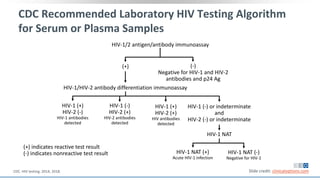
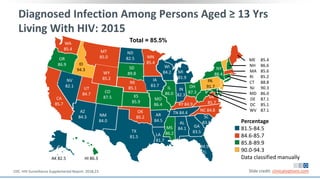
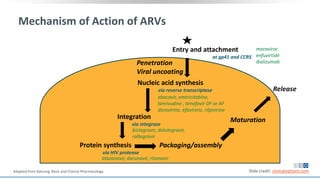
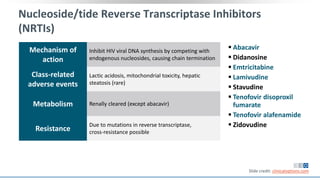
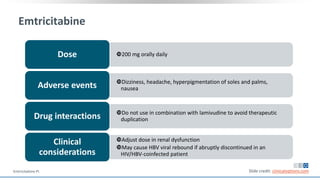
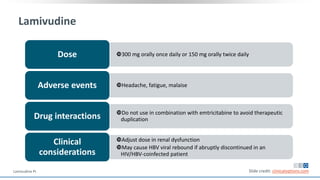
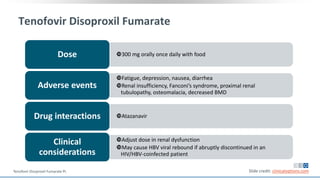
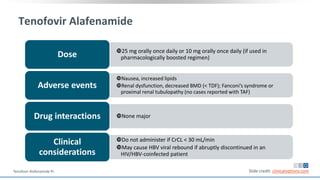
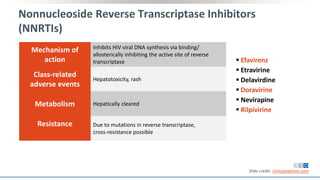
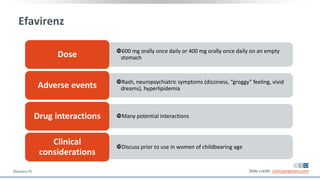
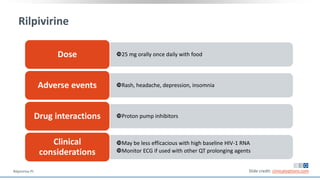
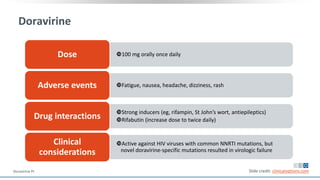
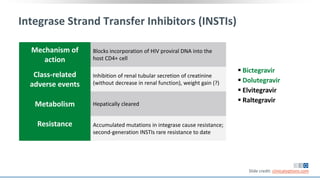
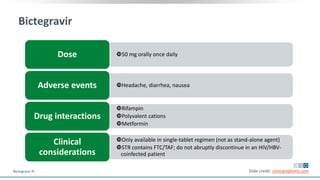
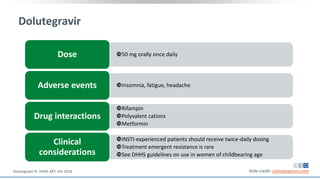
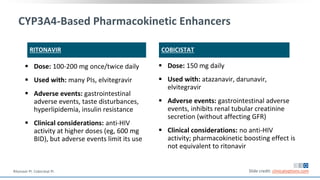
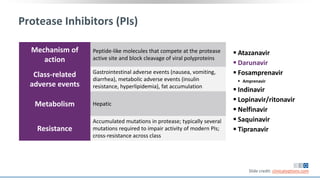
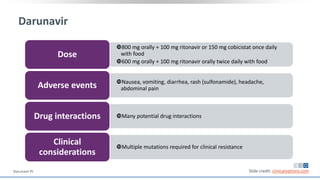
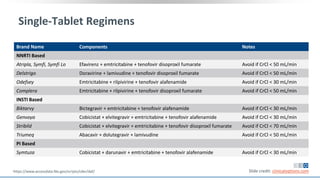
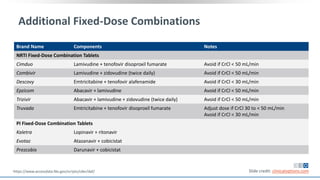
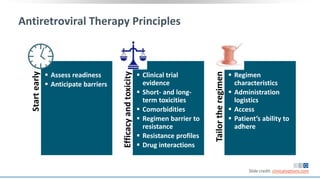
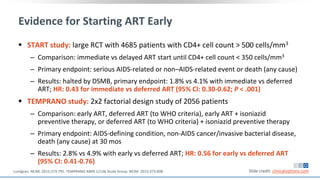
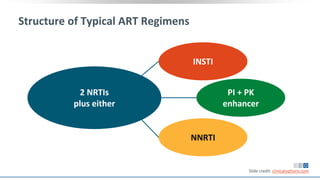
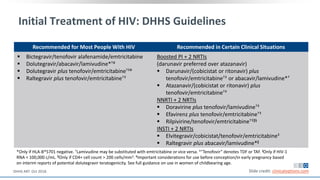
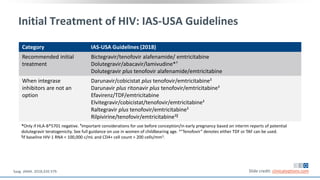
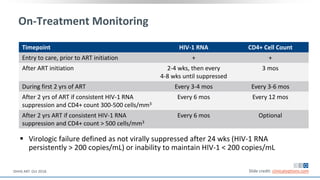
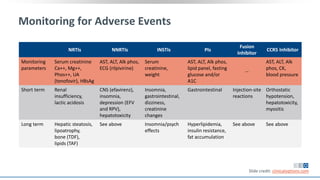
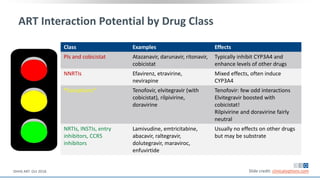
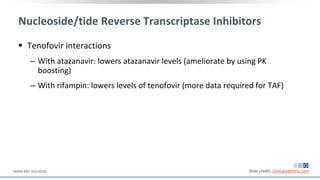
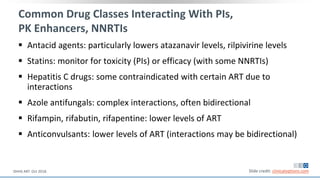
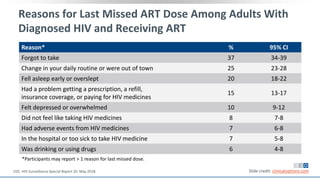
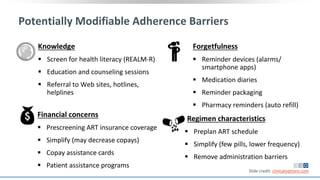
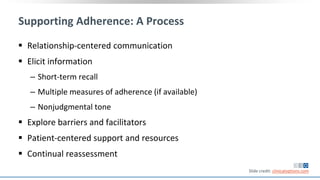
The document discusses pharmacy essentials for HIV screening and management, supported by educational grants. It includes data on the prevalence and demographics of HIV in the U.S., risk assessment, testing recommendations, and details about antiretroviral pharmacology. Key topics also include treatment adherence, drug interactions, and the societal influences on the HIV epidemic.