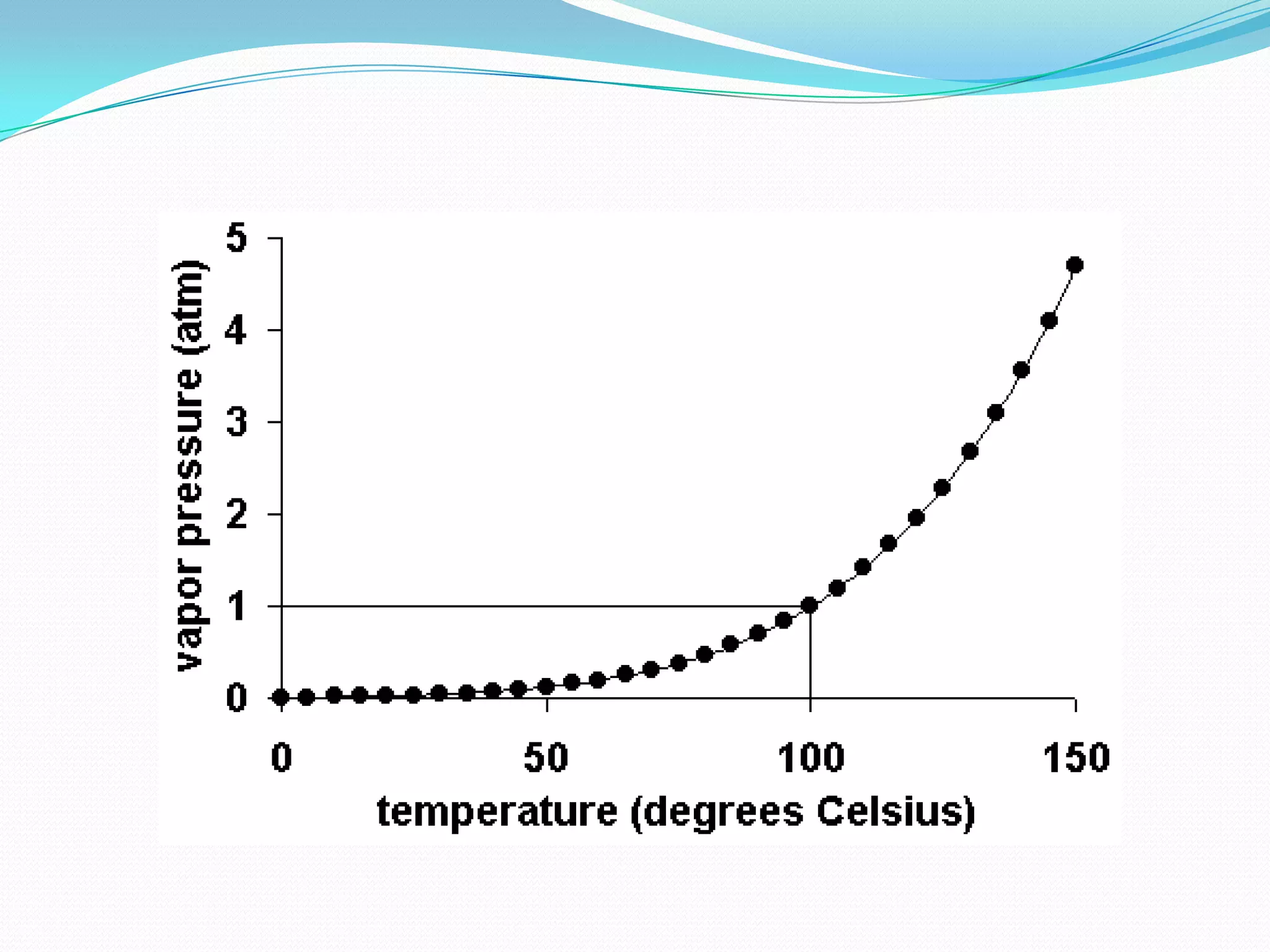
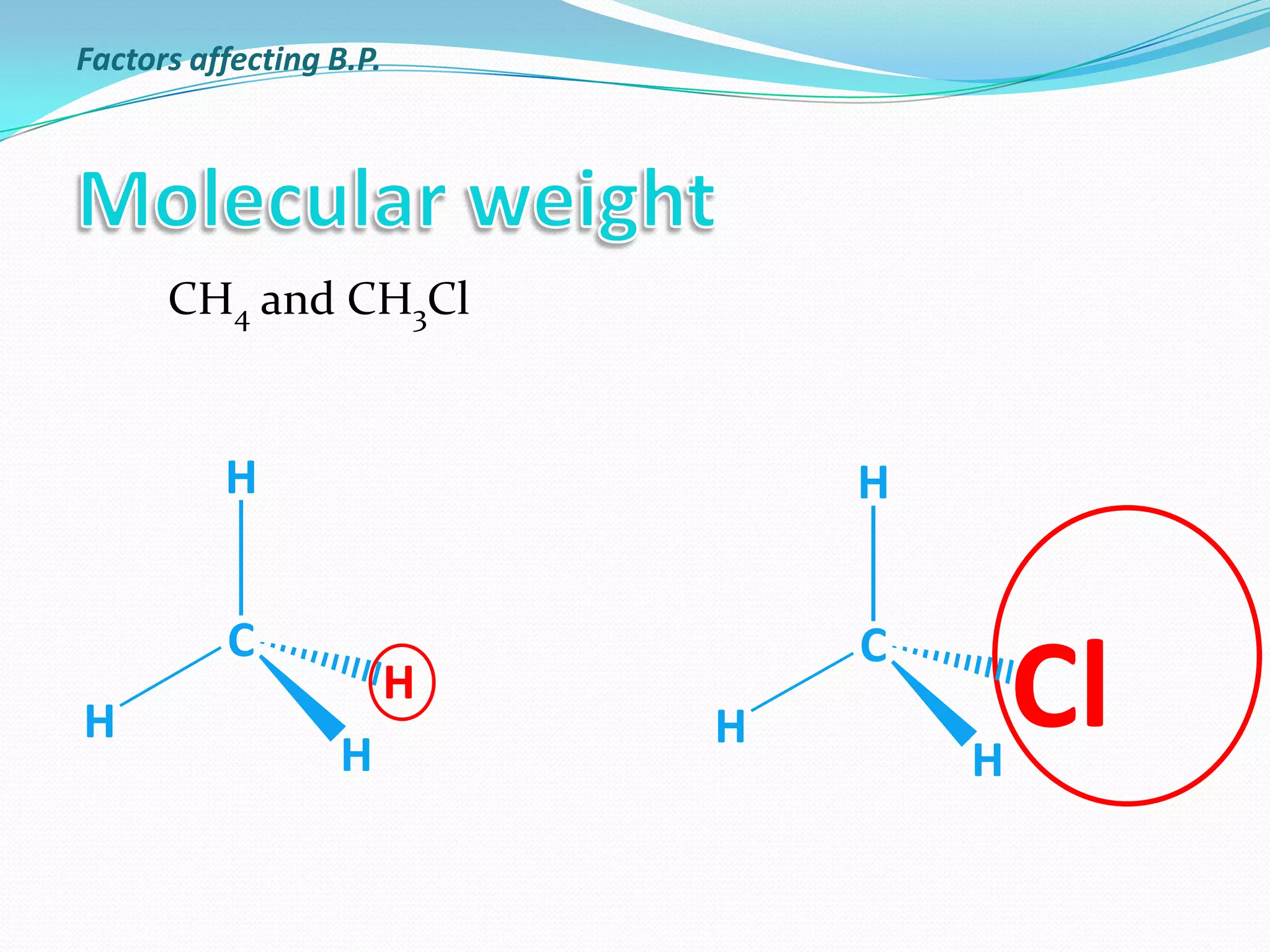
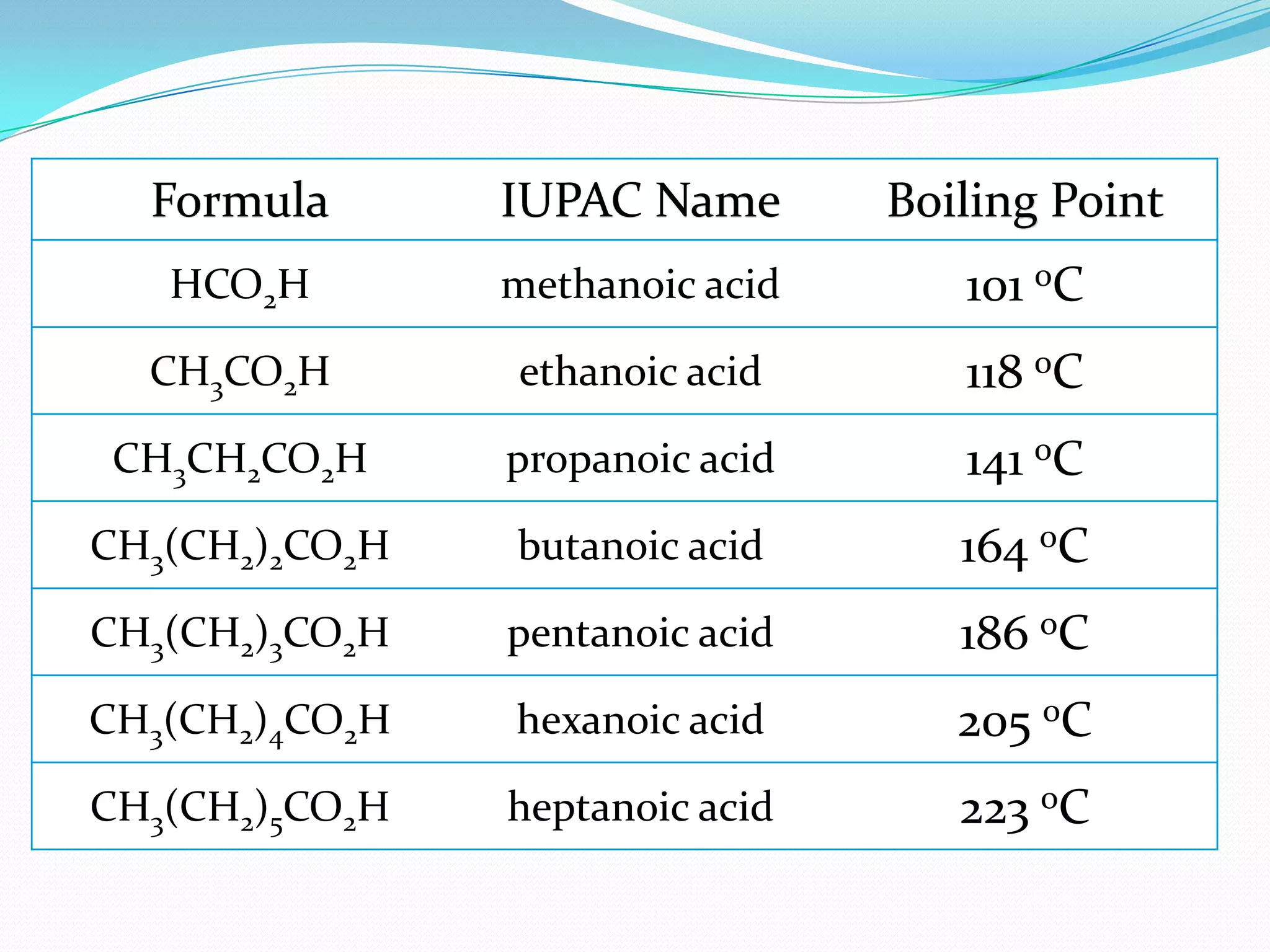
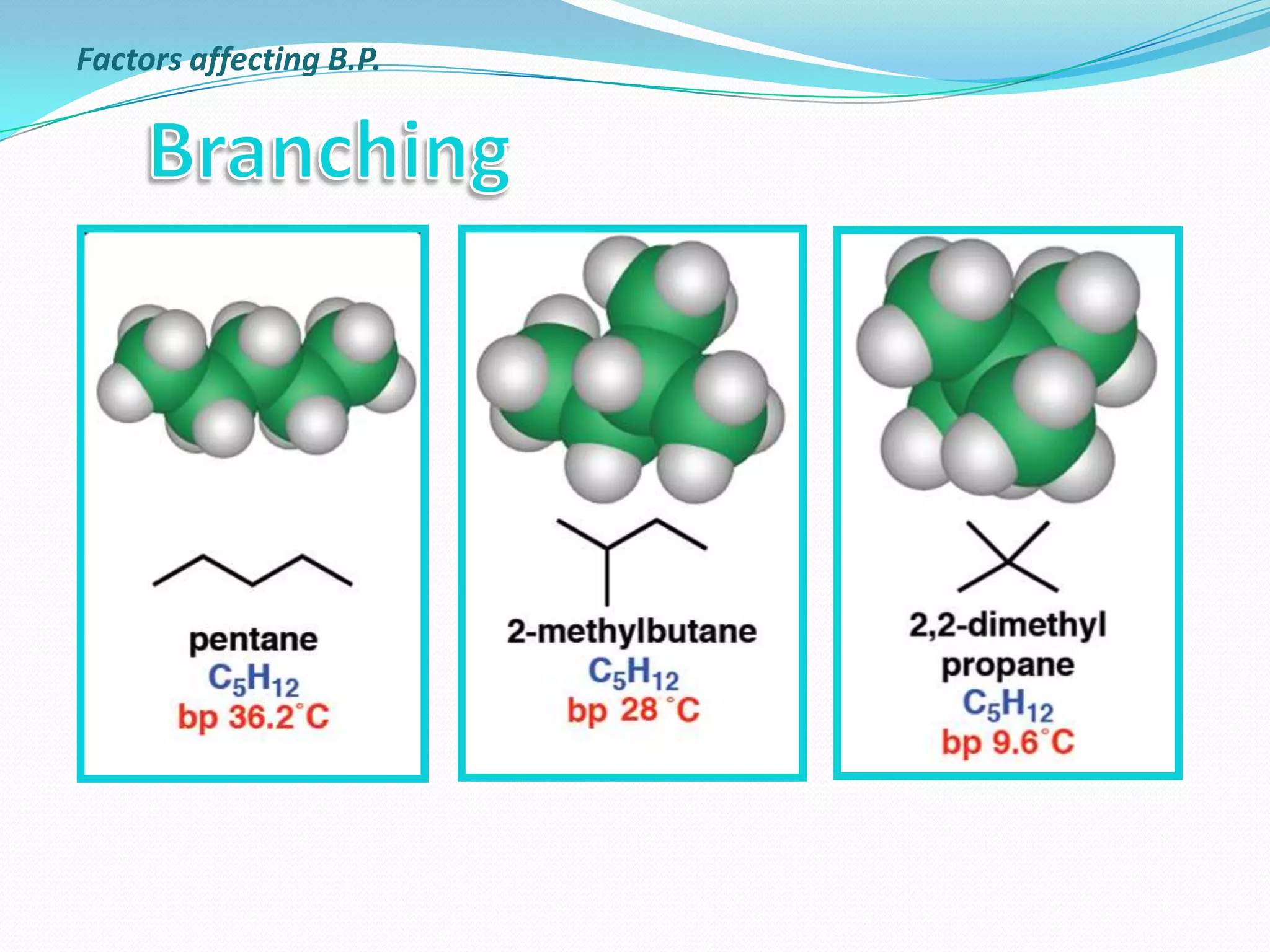
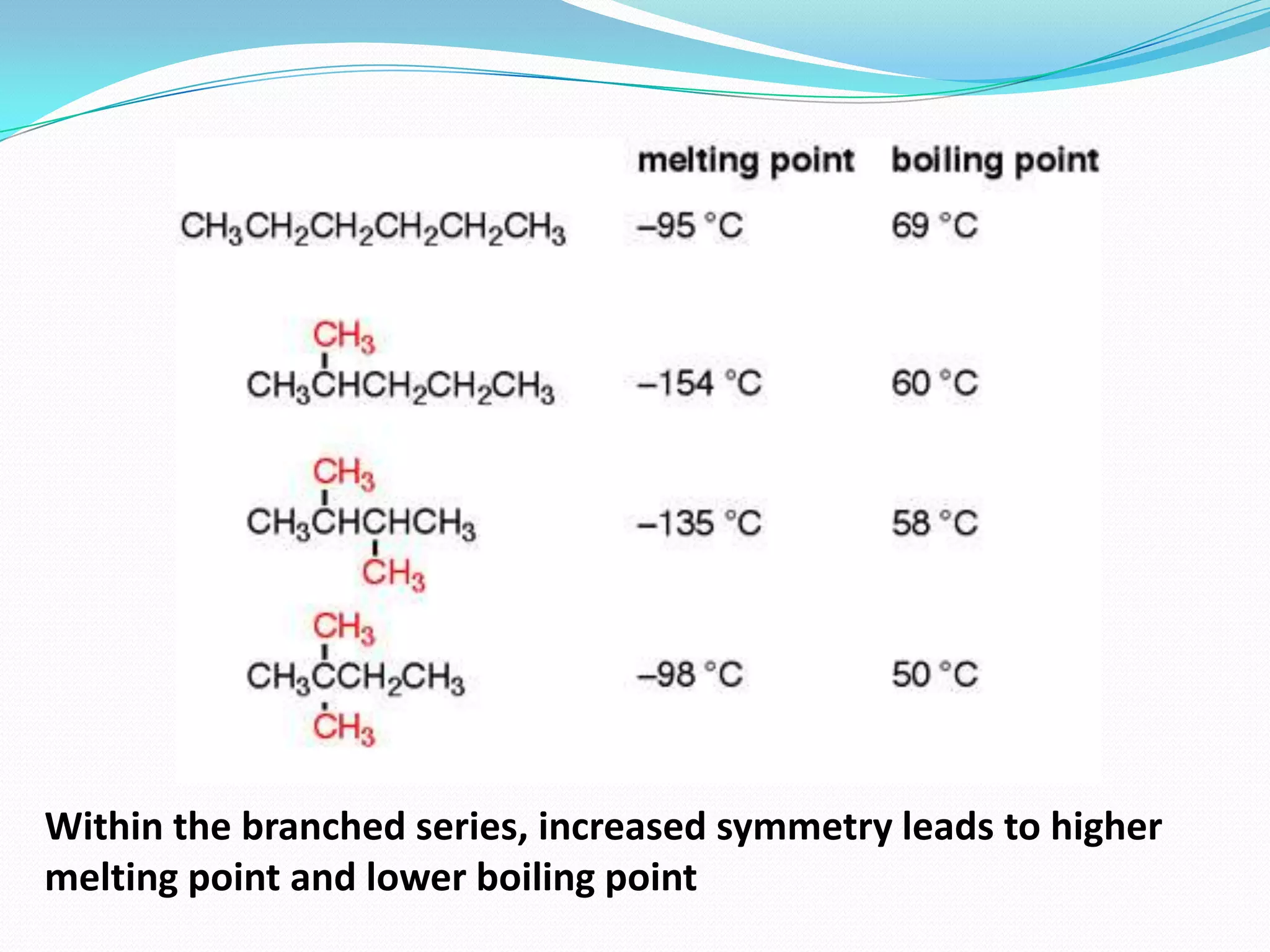
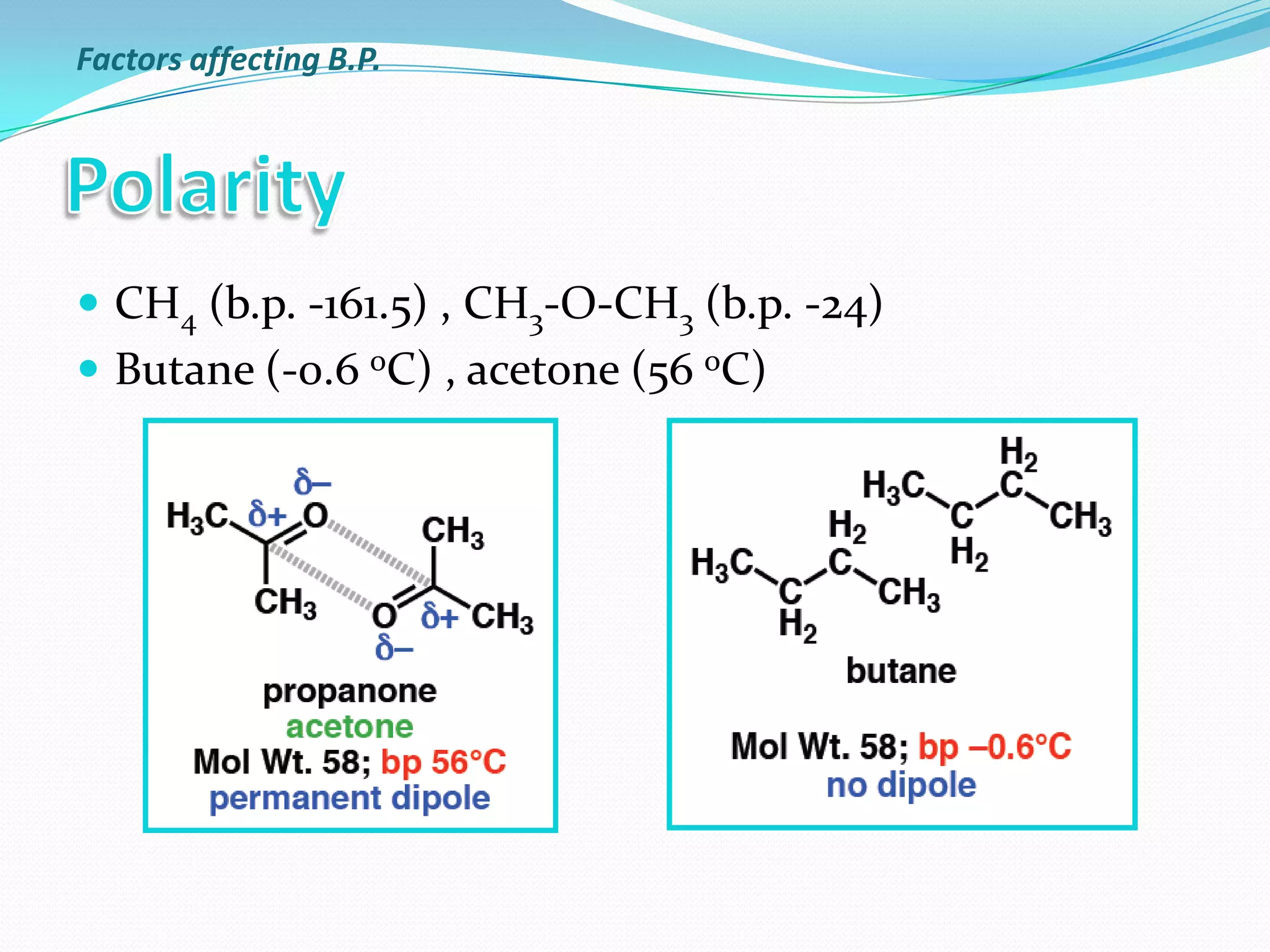
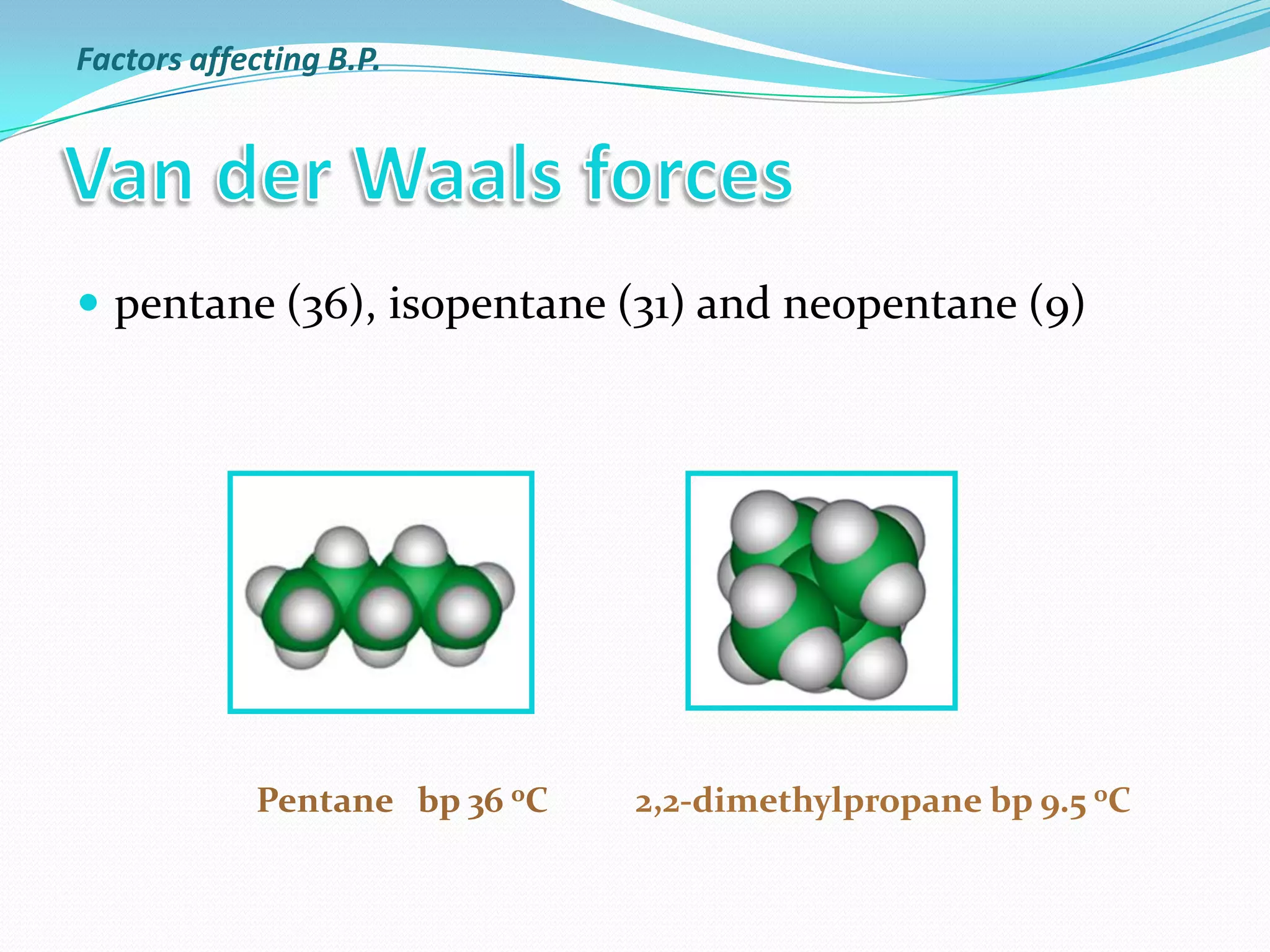
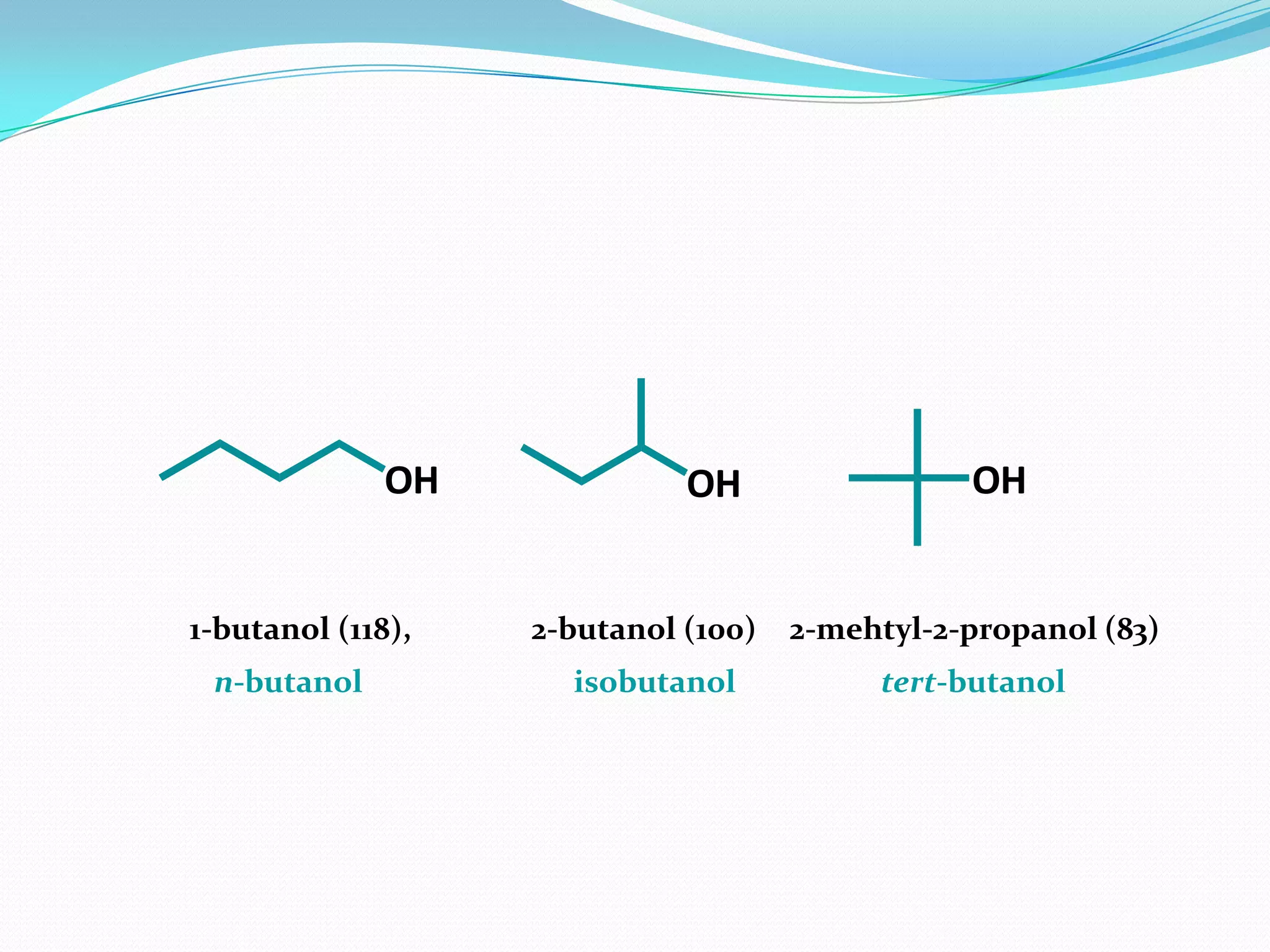
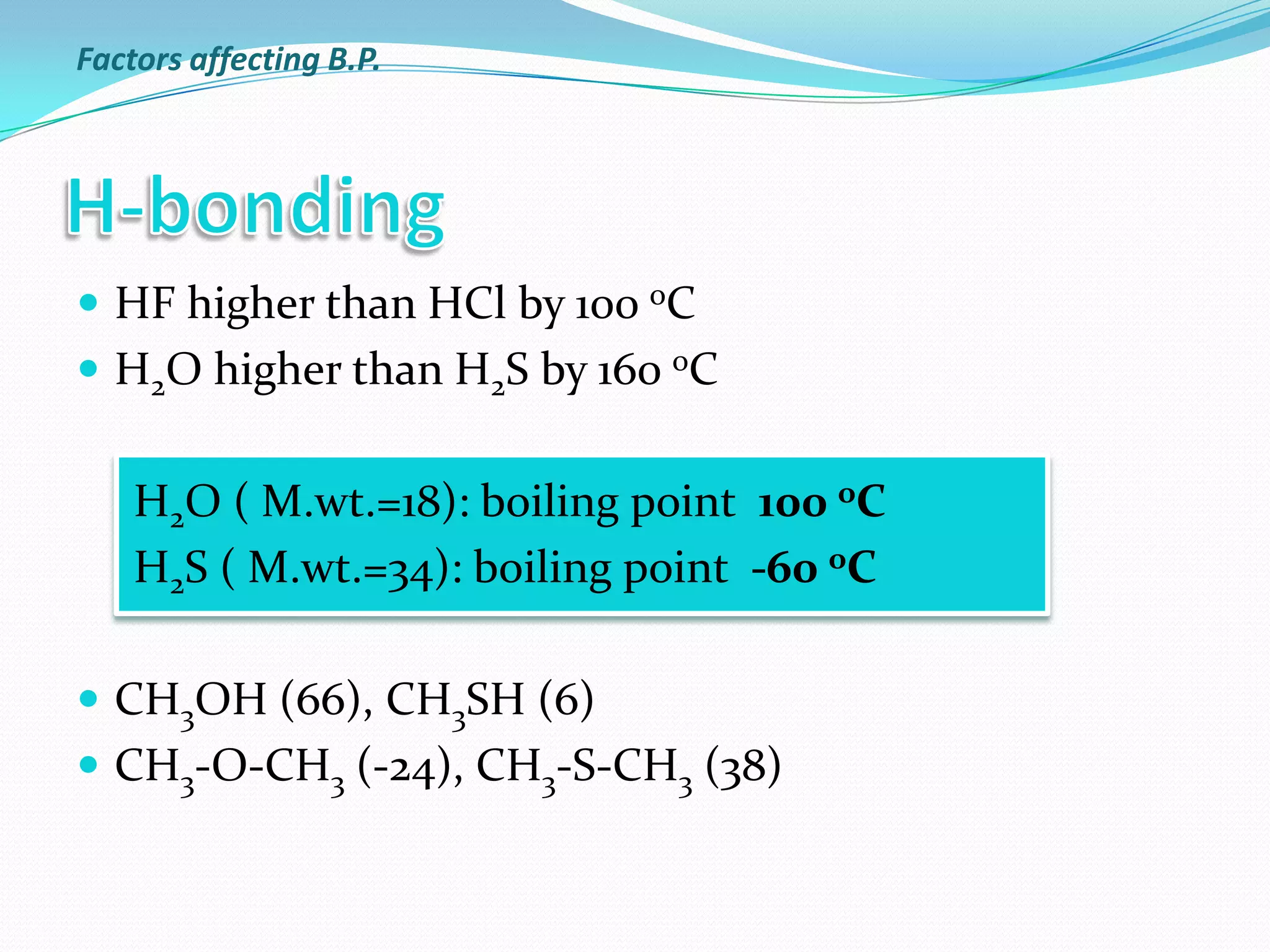
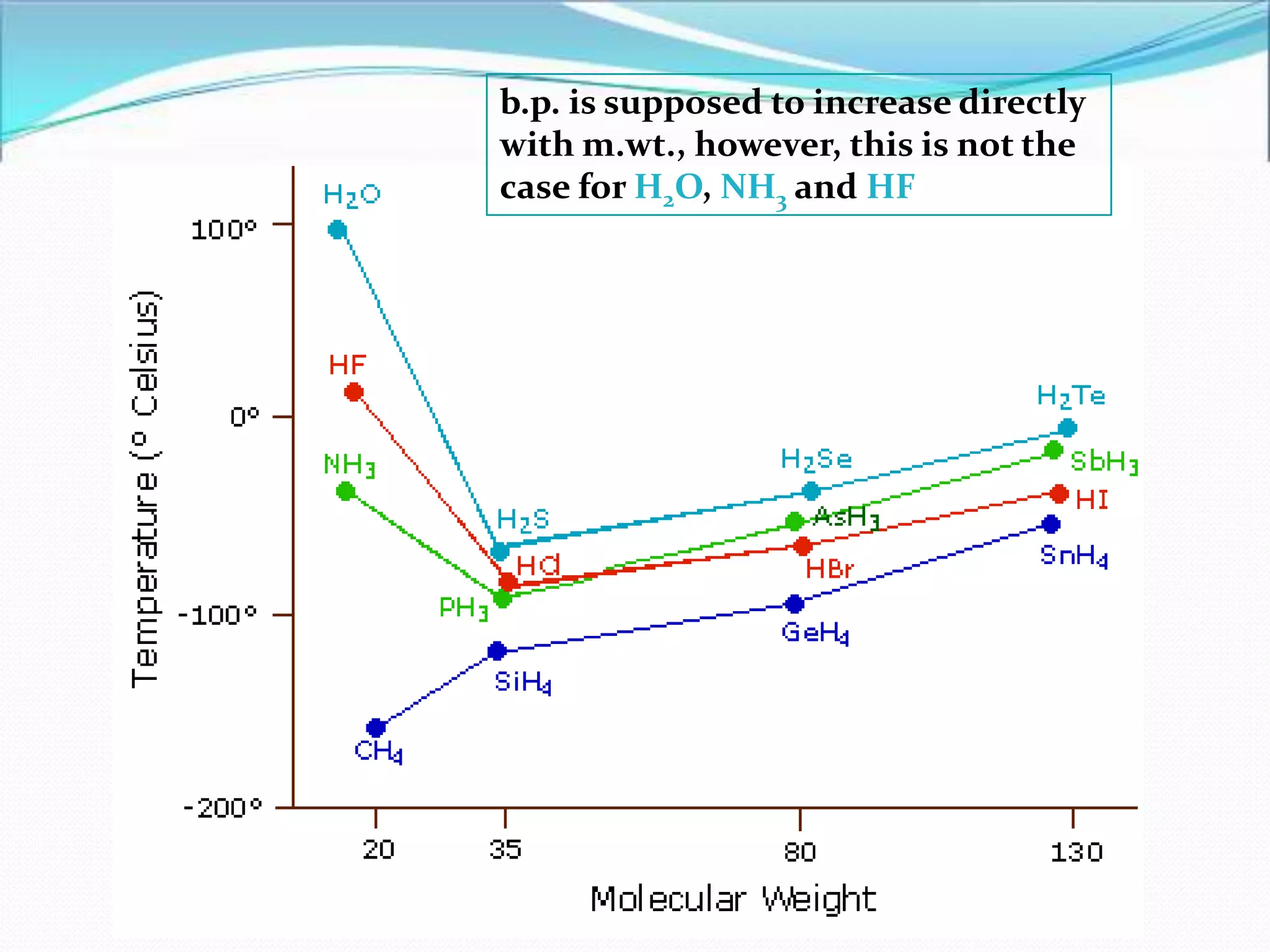
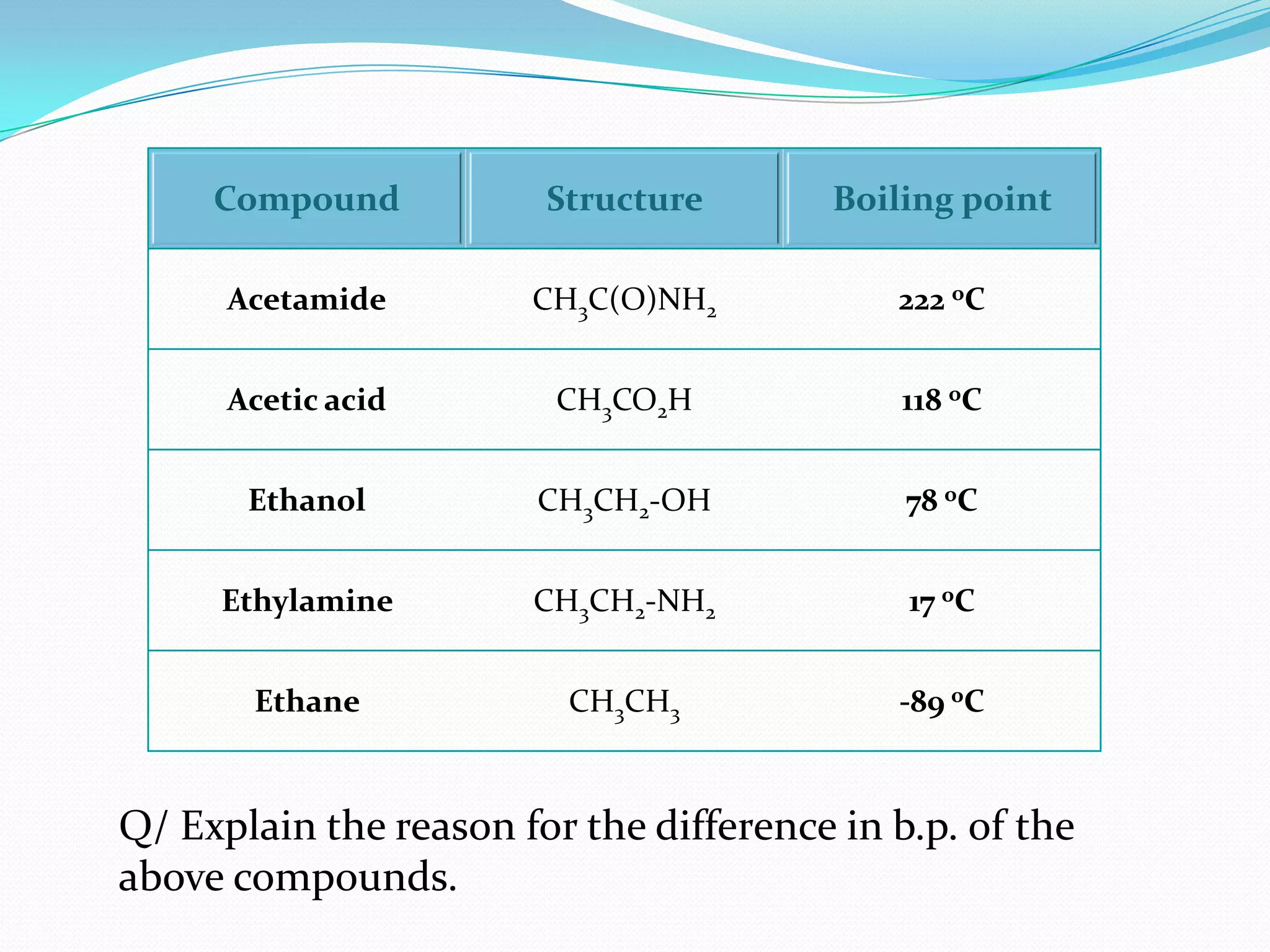
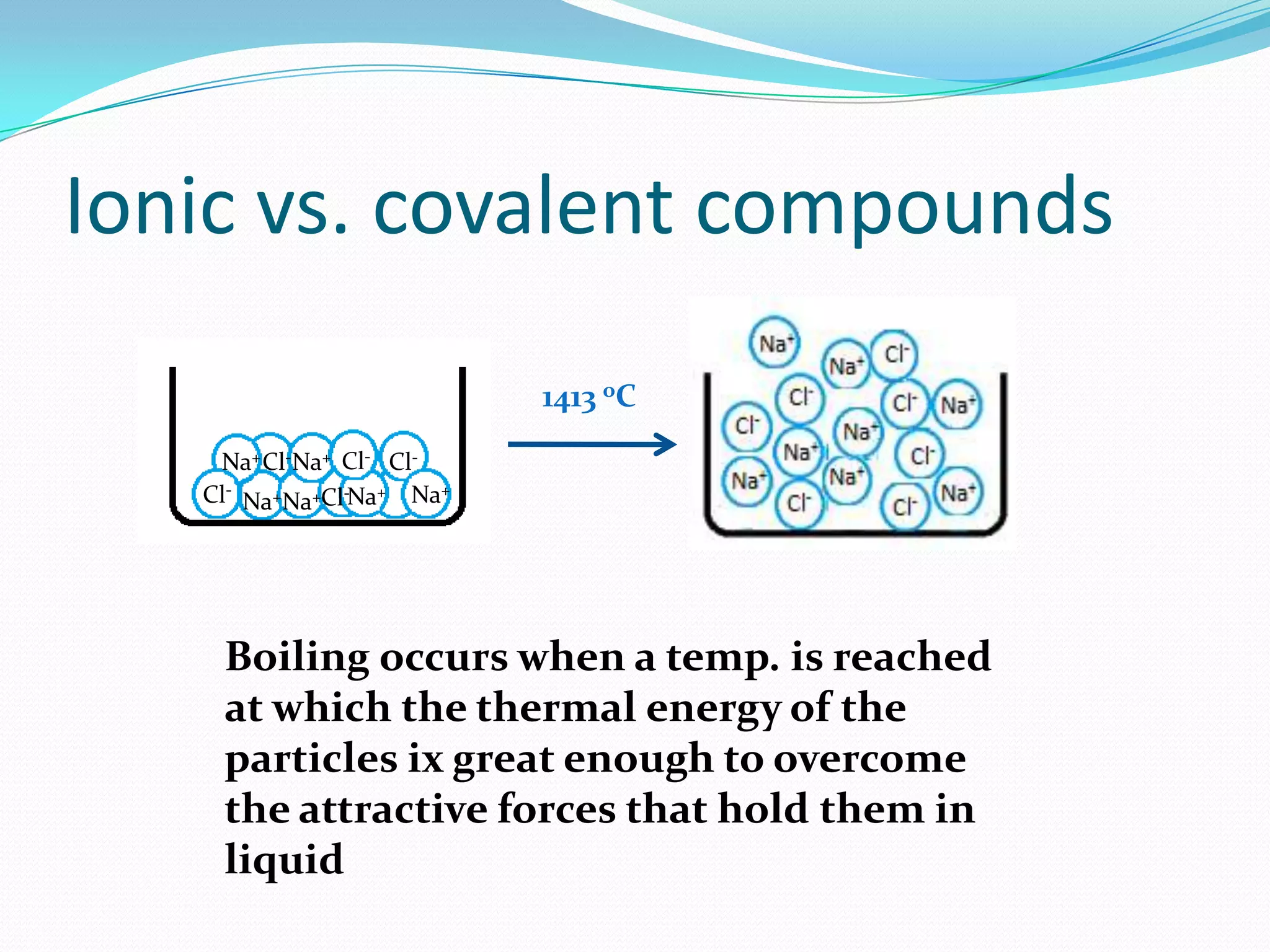
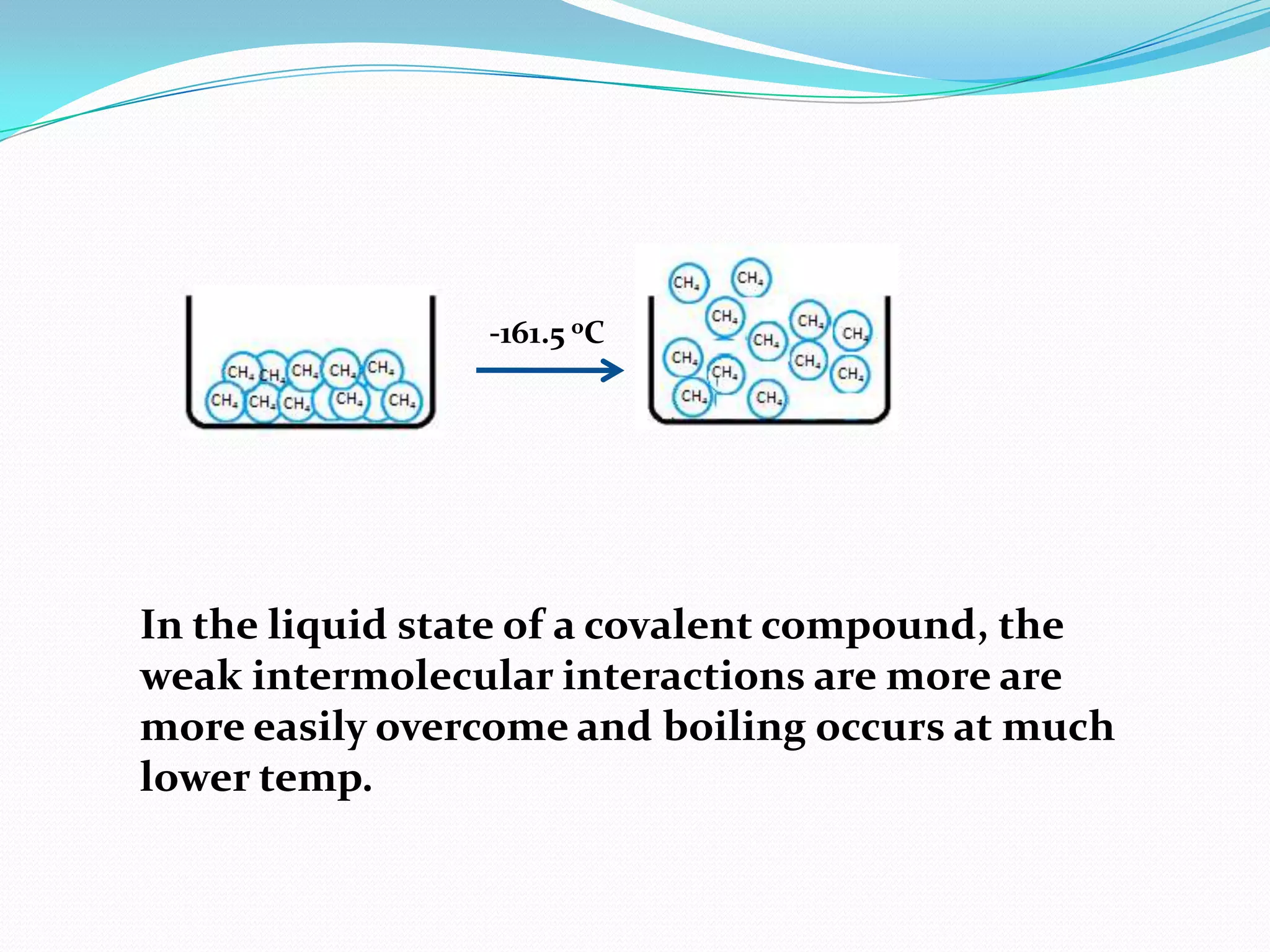
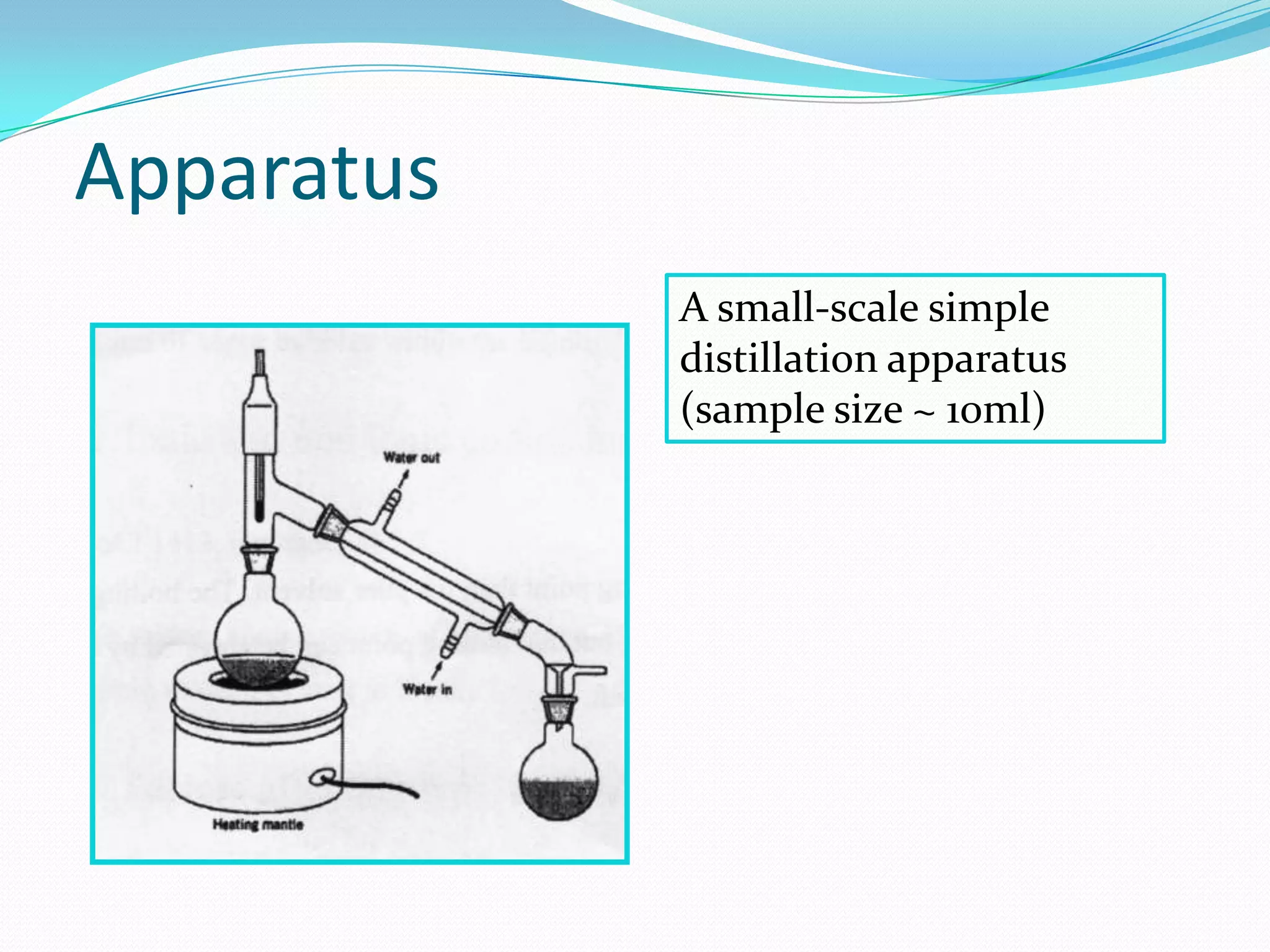
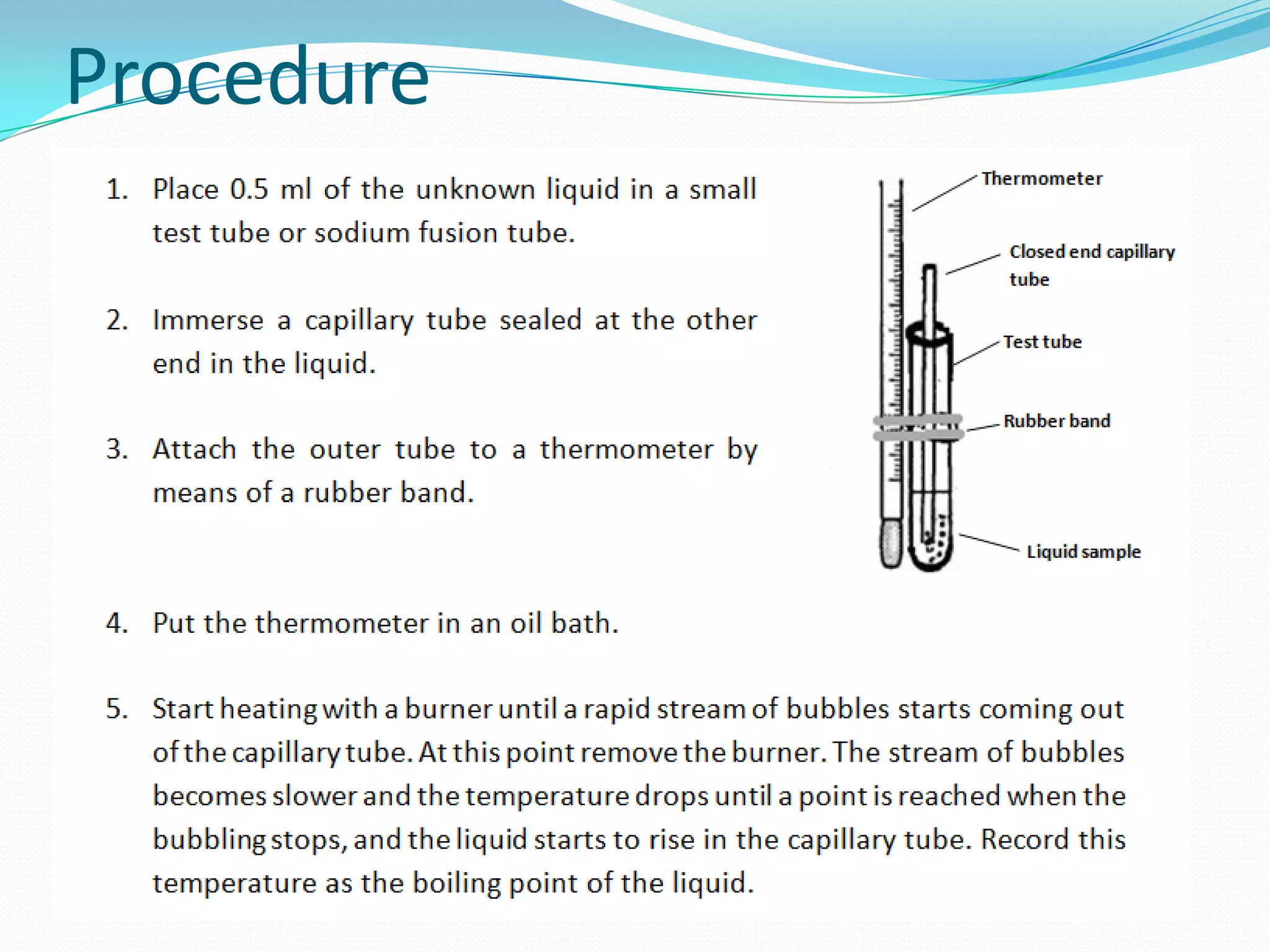
The document discusses the concept of boiling point (b.p.), defining it as the temperature at which a liquid's vapor pressure equals the external pressure, and indicating that b.p. is influenced by factors such as pressure, molecular weight, and intermolecular interactions. It also highlights the significance of b.p. in the identification and purification of compounds and explains how impurities and solutes can affect the b.p. of solutions. Additionally, various examples are provided to illustrate the principles affecting b.p. among different chemical compounds.