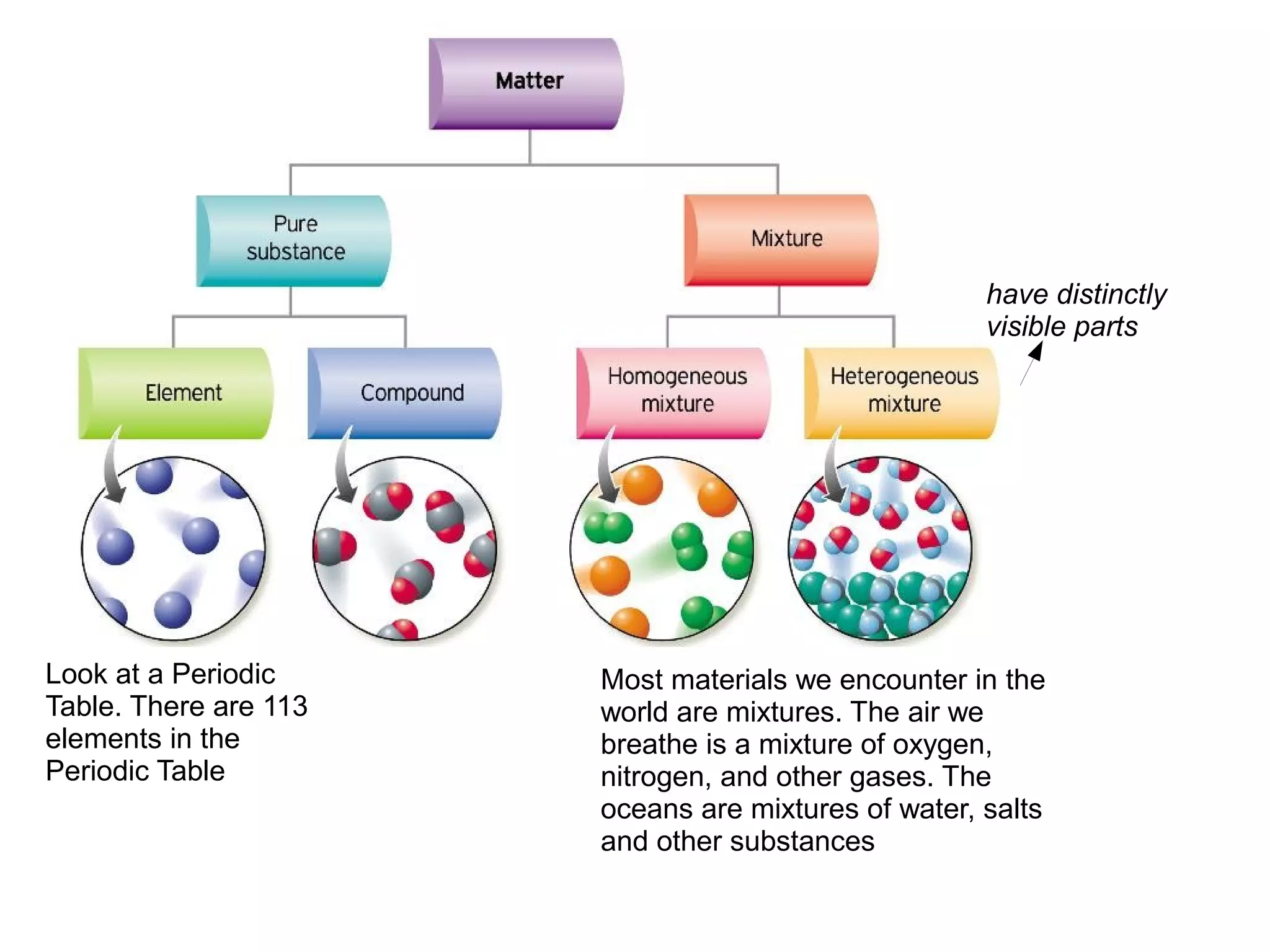
This document discusses the differences between pure substances, mixtures, compounds, and elements. It states:
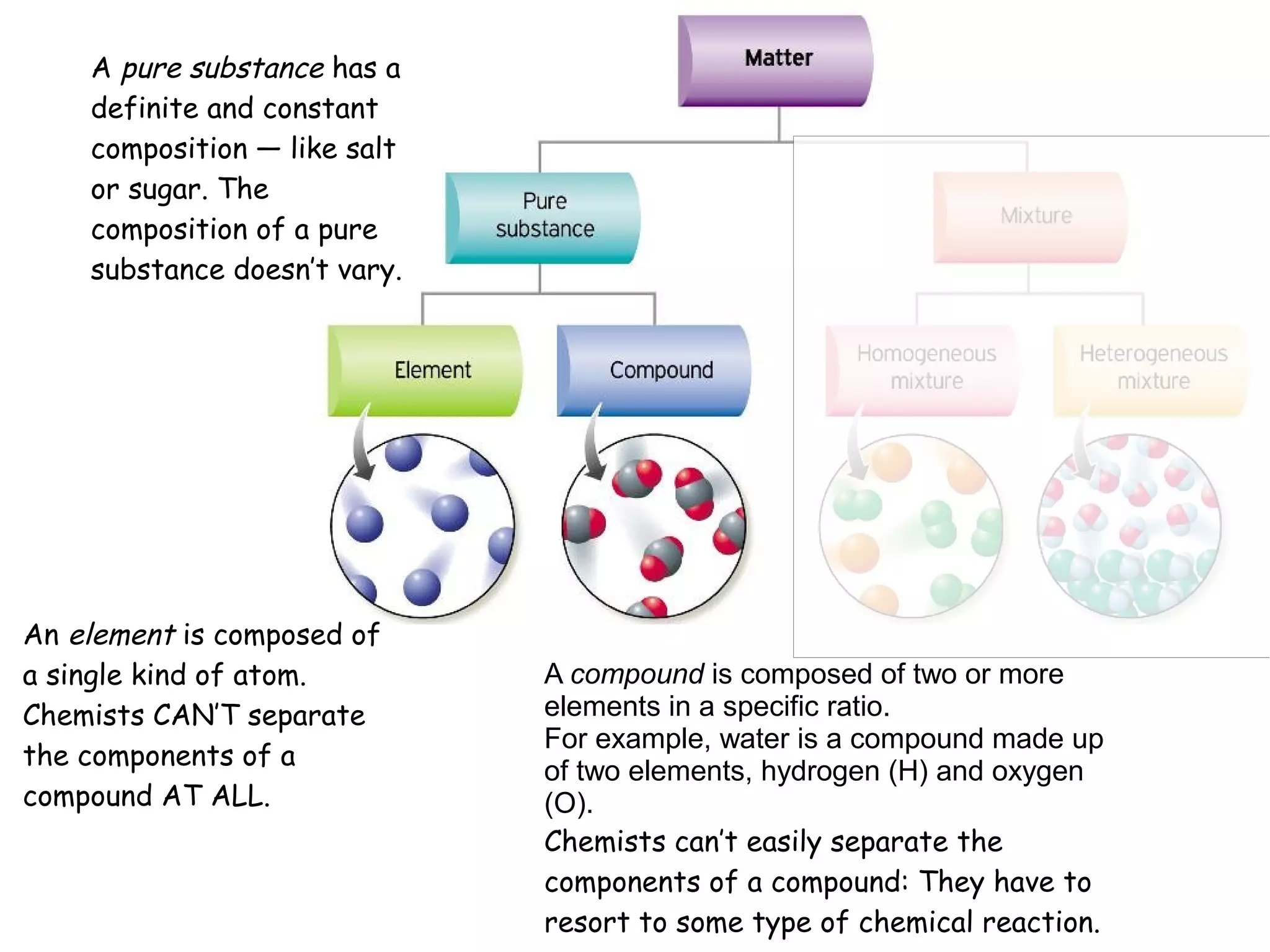
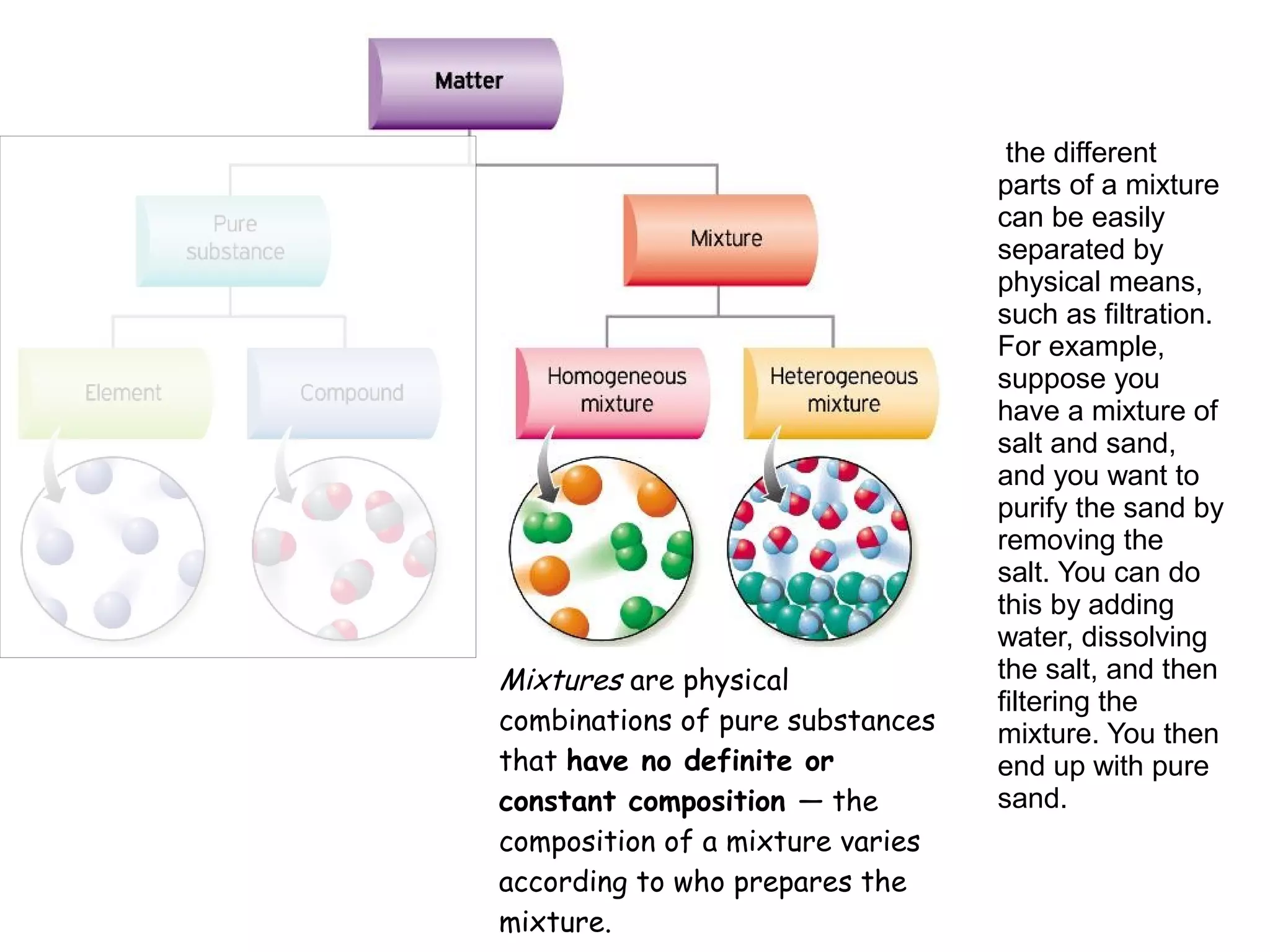
1) A pure substance has a definite composition that does not vary, while a mixture's composition varies depending on who prepares it.
2) An element is made of a single type of atom, while a compound is made of two or more elements in a specific ratio that chemists cannot easily separate.
3) The different parts of a mixture can be easily separated through physical means like filtration.