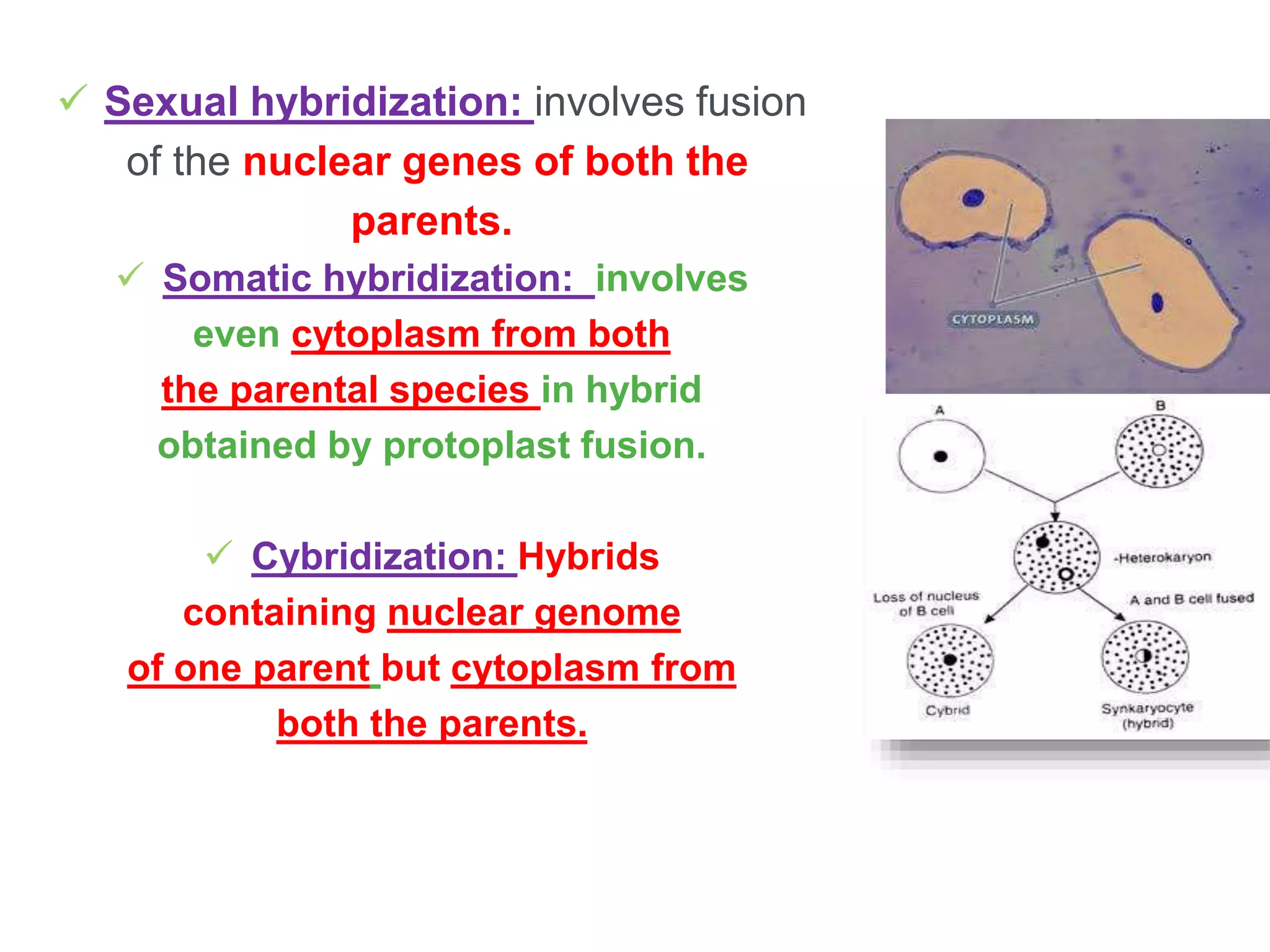
Somatic hybridization involves the fusion of isolated plant protoplasts to generate hybrid cells and plants combining the genetic material of both parental species. It allows for the production of novel interspecific and intergeneric hybrids not achievable through sexual hybridization. The key steps are isolating protoplasts using enzymatic methods, fusing the protoplasts using techniques like PEG or electrofusion, selecting hybrid cells, culturing the hybrid cells, and regenerating hybrid plants. Somatic hybridization has various applications in plant breeding and crop improvement.