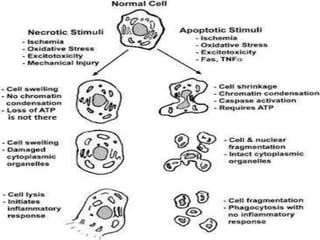
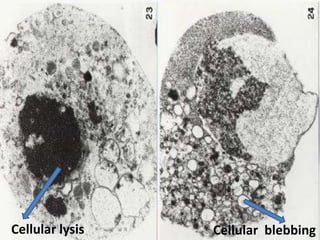
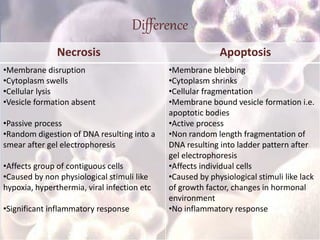
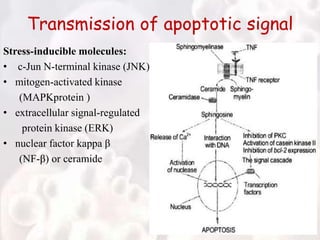
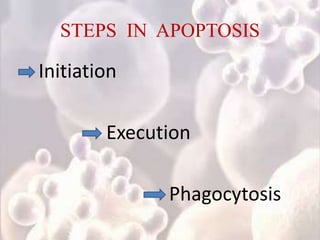
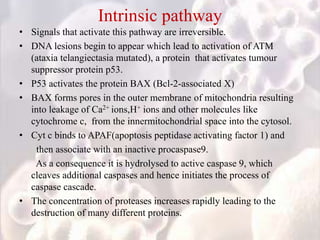
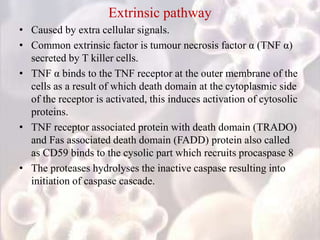
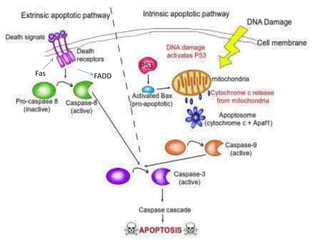
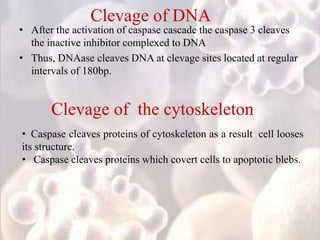
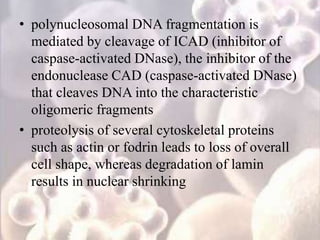
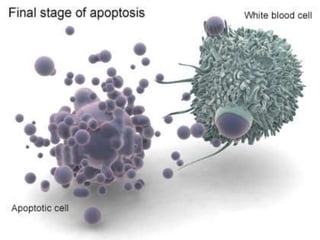
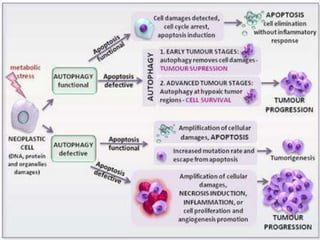
Every cell has a natural life cycle involving birth and death. There are two main types of cell death: necrosis and apoptosis. Necrosis is accidental cell death due to external injury, while apoptosis is a carefully regulated process in which cells play an active role in their own death. During apoptosis, cells shrink, break into fragments, and are phagocytosed without causing inflammation. Precisely regulated apoptosis is important for normal development, immune function, and homeostasis, while defects can lead to diseases. Many cancer therapies aim to trigger the apoptosis pathway in tumor cells.