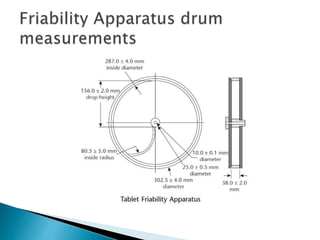
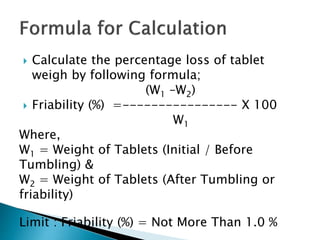
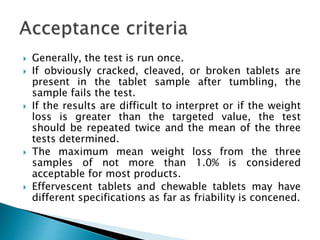
Friability testing involves placing a sample of tablets into a drum that rotates at 25 rpm for 100 revolutions. The tablets are weighed before and after the test to determine any weight loss due to mechanical stress. An acceptable friability is less than 1% weight loss, as this ensures tablets can withstand forces during manufacturing, distribution, and handling by customers. The test is run using either a single or double drum friabilator, following procedures for sample size, rotation speed, and calculation of percentage weight loss specified in pharmacopeia.