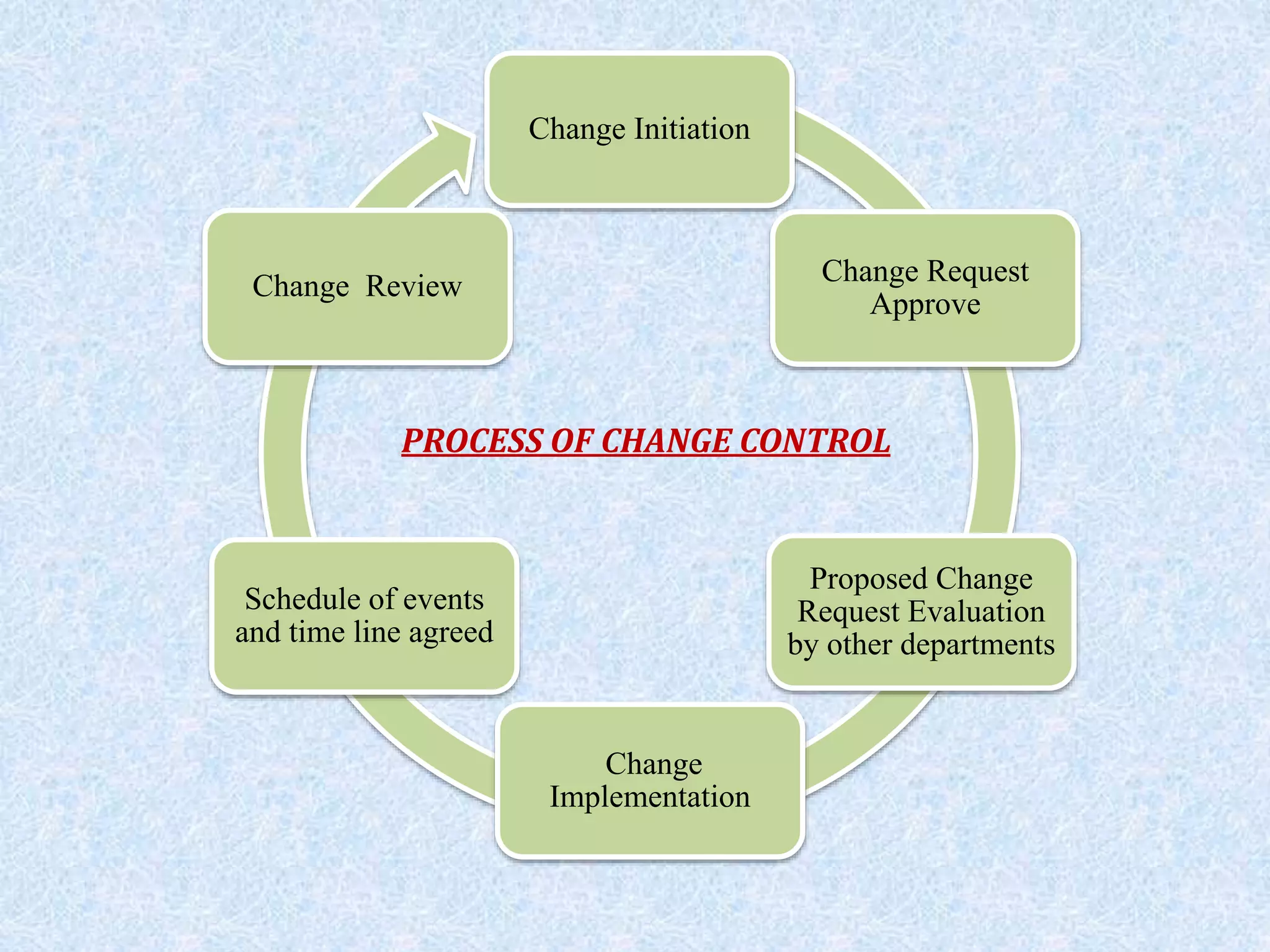
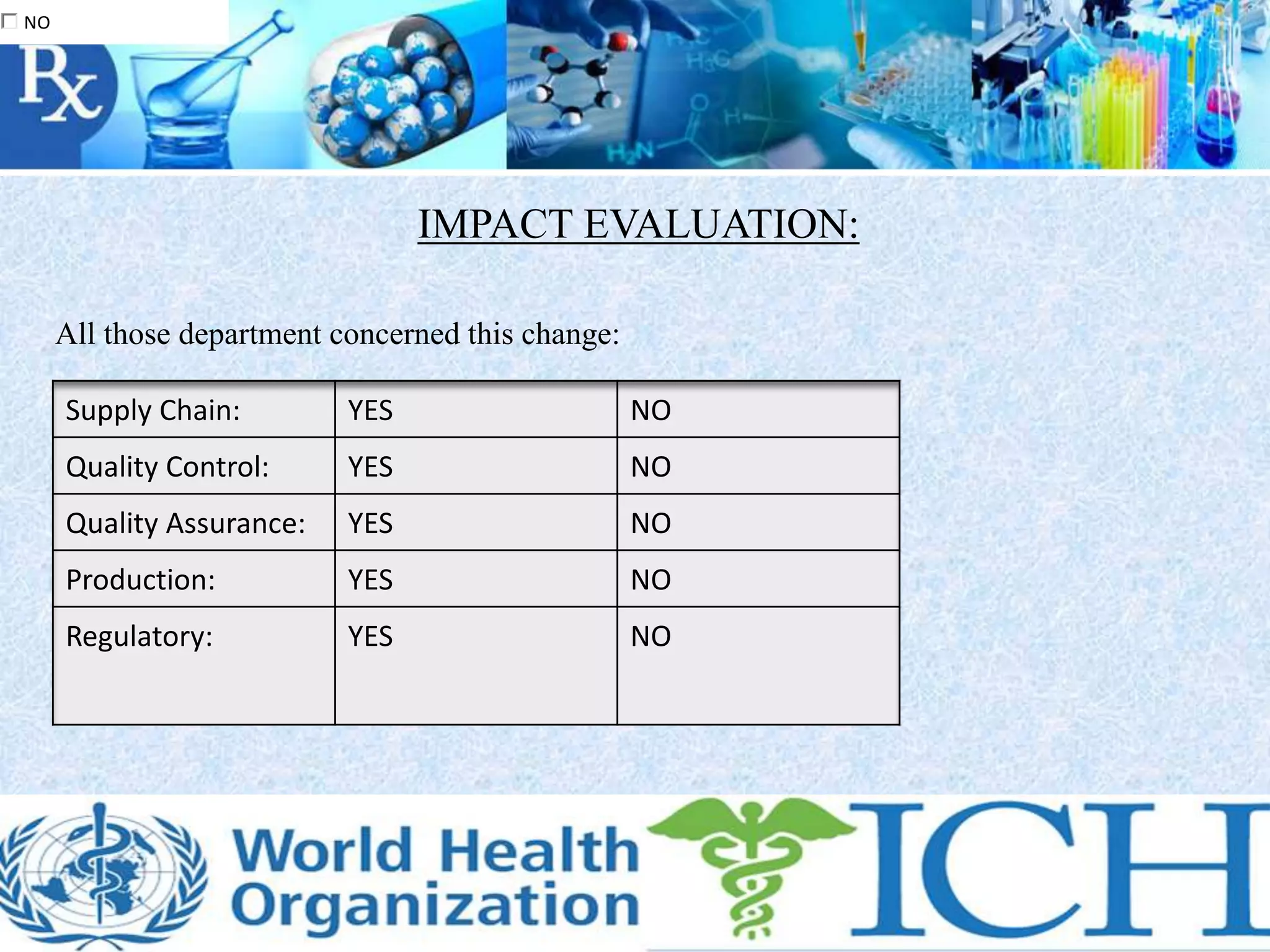
The document discusses a proposed change in the coating process for Dapakan 500mg film coated tablets from a solvent coating to an aqueous coating. It describes changing from coating with Opadry OIC 7000 to coating with Opadry II. A risk assessment is proposed to evaluate any changes in color, weight gain, thickness or process validation needs. The impact on materials management, quality control, quality assurance, production and regulatory requirements is evaluated. References from regulatory bodies on quality guidelines and GMP are also provided.