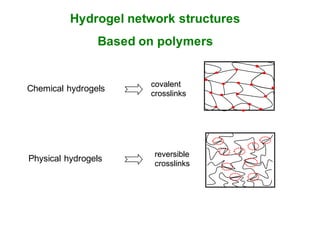
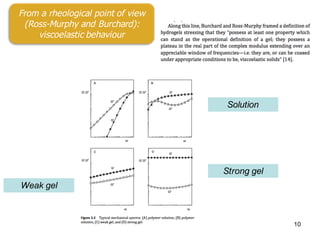
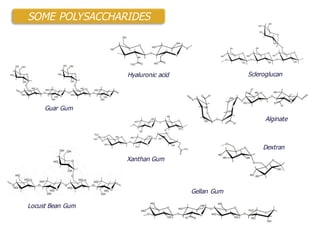
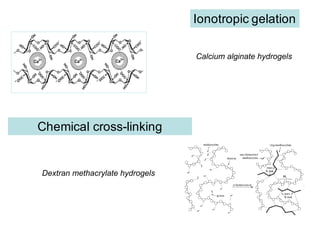
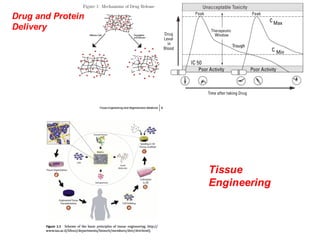
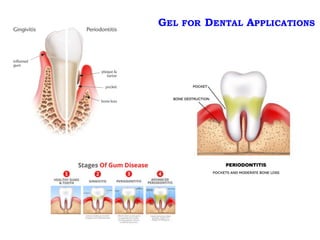
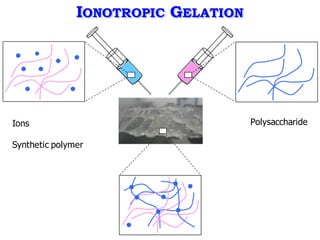
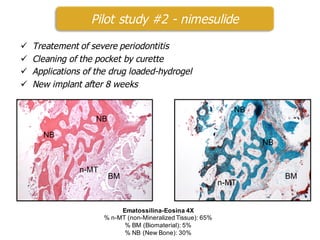
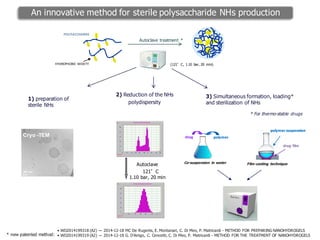
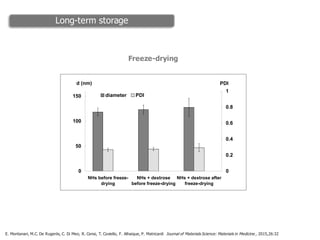
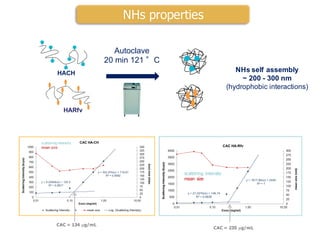
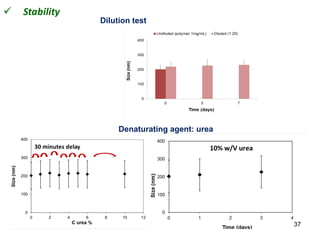
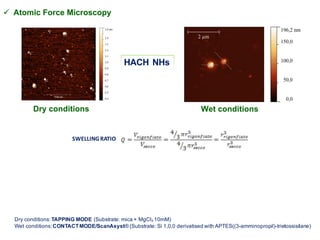
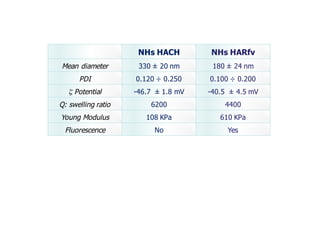
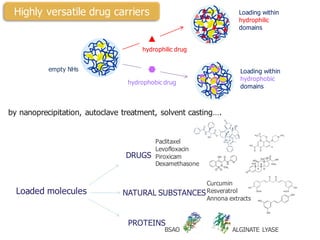
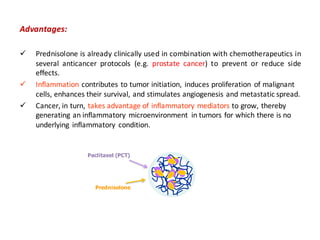
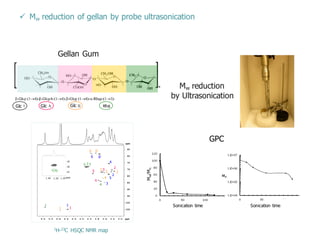
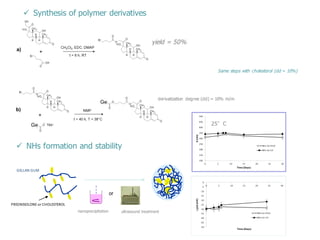
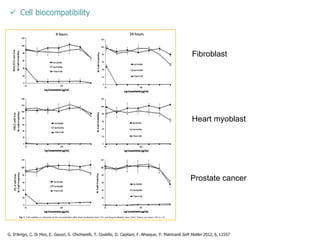
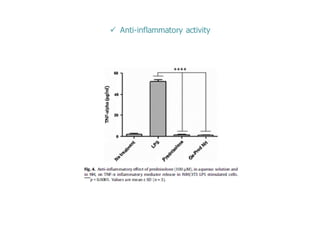
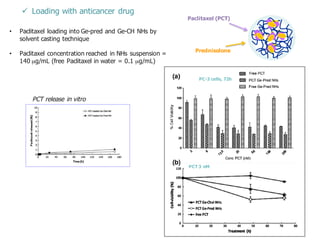
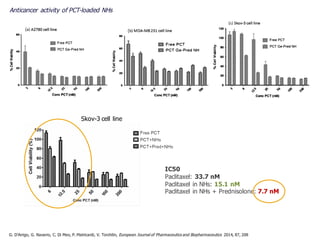
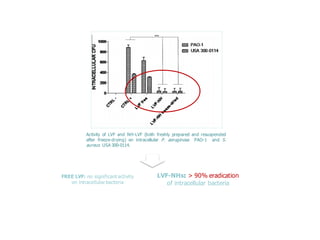
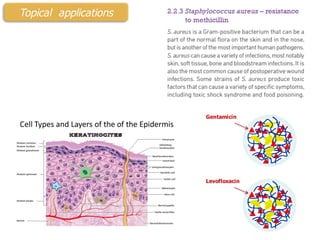
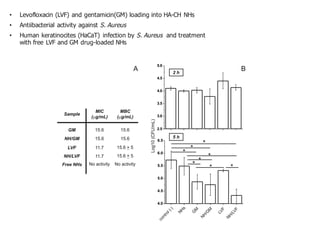
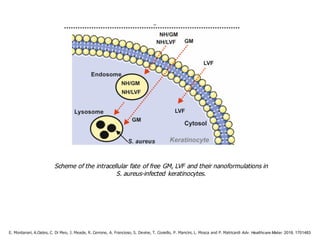
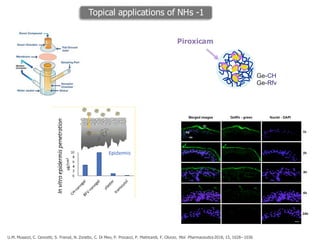
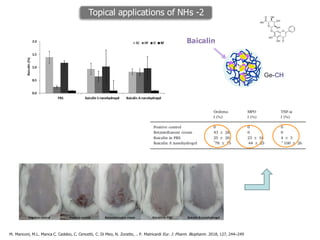
This document summarizes a presentation about polysaccharide hydrogels and their applications. It discusses how polysaccharides can be used to form hydrogels through various methods like ionotropic gelation or chemical cross-linking. It then outlines several biomedical applications of polysaccharide hydrogels, including for drug and protein delivery, tissue engineering, dental applications, and heritage protection. The document also discusses using polysaccharide nanohydrogels for controlled drug delivery and how they can be loaded with various drugs and biological agents.