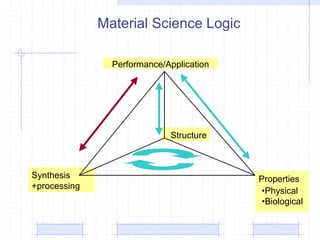
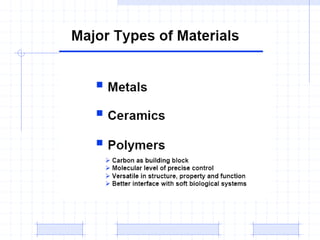
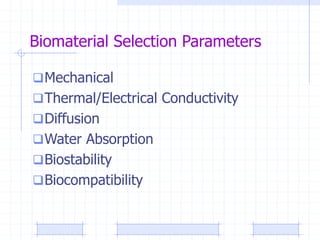
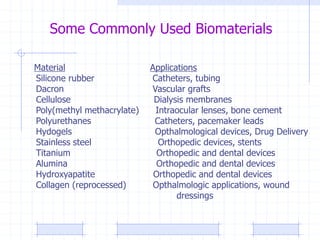
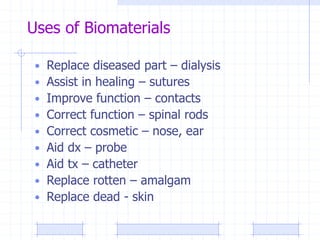
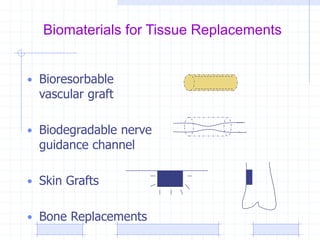
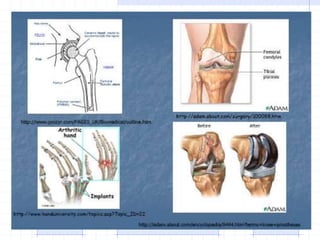
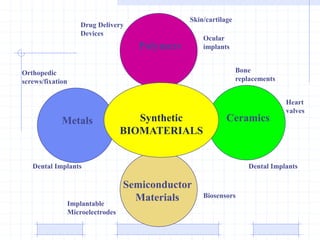
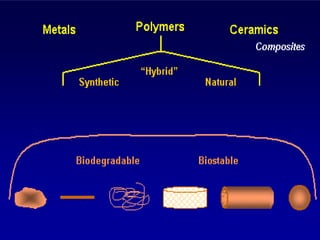
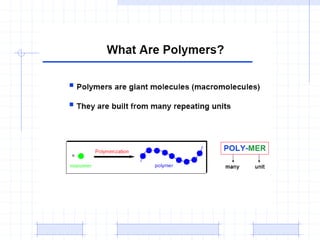
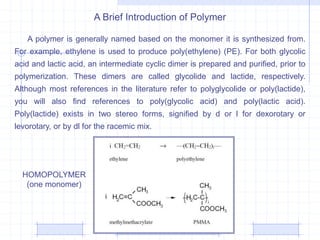
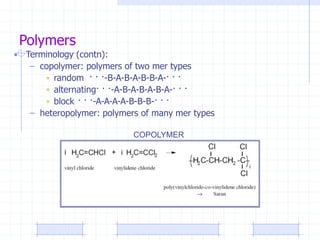
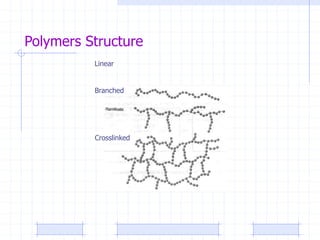
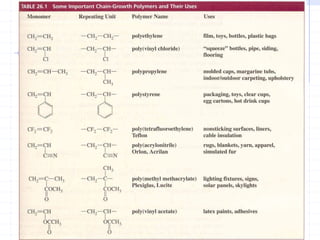
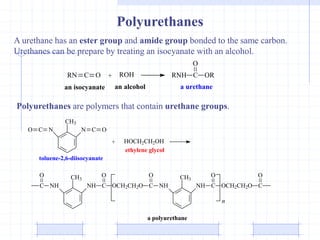
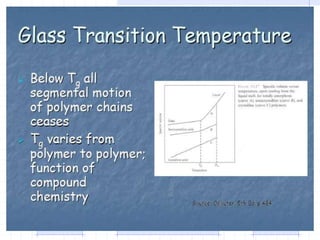
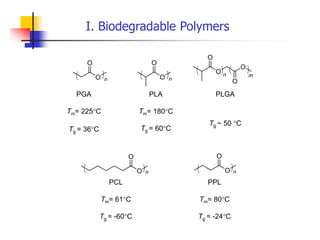
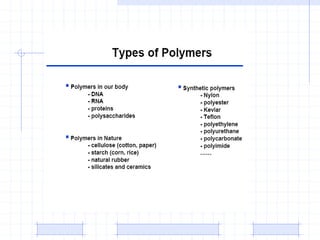
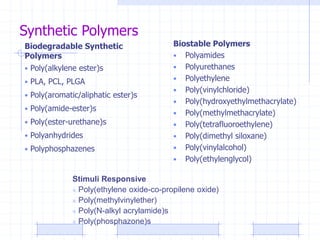
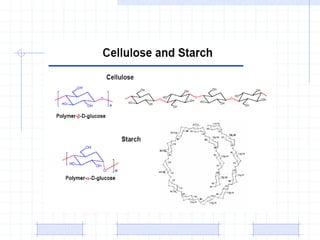
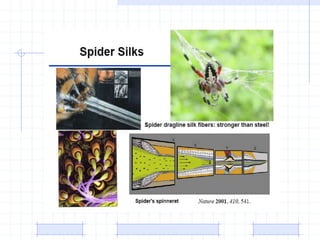
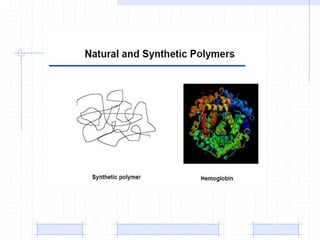
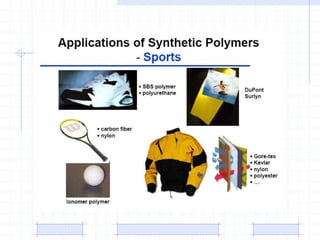
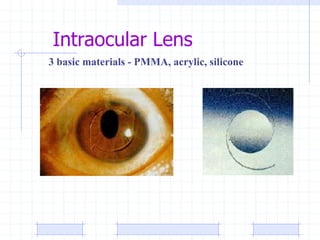
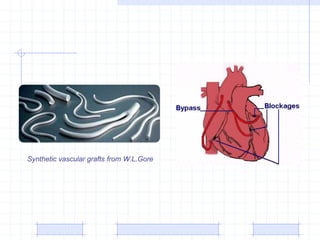
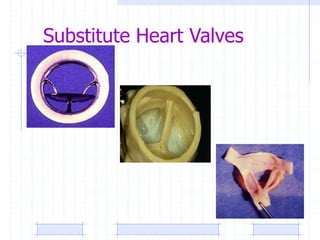
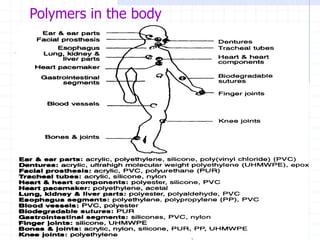
The document discusses synthetic biomaterials and polymers used in medicine. It provides definitions for biomaterials and biocompatibility. Biomaterials are materials designed for use inside the body, and their interaction with biological systems is studied. The document outlines commonly used biomaterial classes including metals, ceramics, polymers, composites and hydrogels. Examples are given of materials used for applications like orthopedic and dental implants, vascular grafts, and drug delivery devices. Key considerations for biomaterial selection like mechanical properties, biostability and biocompatibility are also summarized.