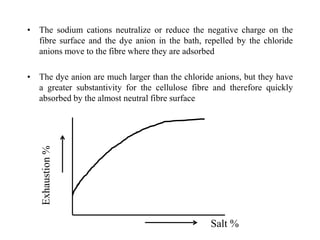
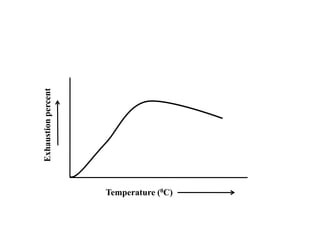
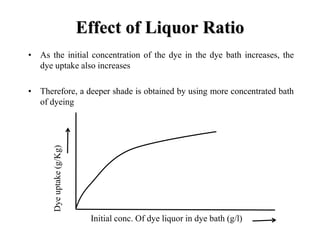
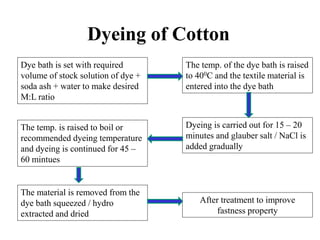
Direct dyes are water-soluble aromatic compounds that have an affinity for cellulose fibers like cotton. They are applied as aqueous solutions and bond to fibers physically through hydrogen bonding and van der Waals forces. Direct dyes generally have poor fastness properties but these can be improved through after-treatments using metallic salts like copper or chromium compounds, or formaldehyde, which increase the dye's molecular weight and bonding strength to the fibers. Key factors that influence direct dye uptake include electrolyte concentration, temperature, liquor ratio, and dye class.