This document discusses direct dyeing processes. It covers:
1. Direct dyes are initially dyed slowly for even dyeing, and factors like heat, salt, and dye properties affect the dyeing rate.
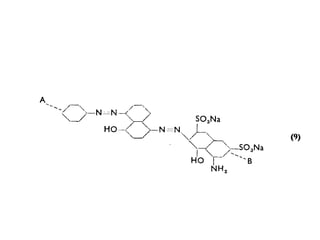
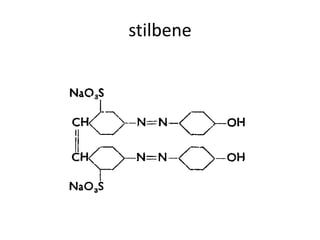
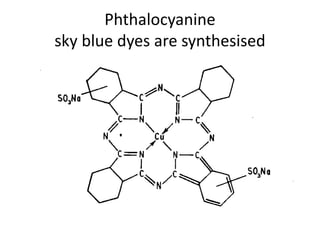
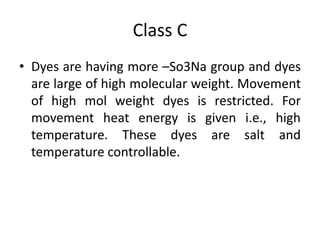
2. Class C dyes are large and have more sulfate groups, so require heat and salt/temperature control for movement during dyeing.
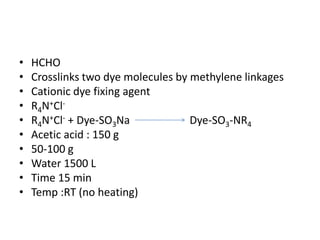
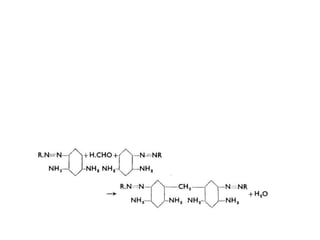
3. The dyeing process involves chemicals like salt, soda ash, and softeners, with machines dyed based on material-liquor ratio and capacity. Proper preparation and application of dyes and after-treatments like fixation are described.