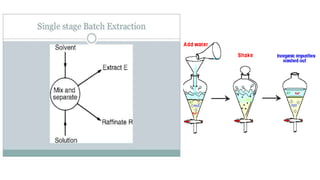
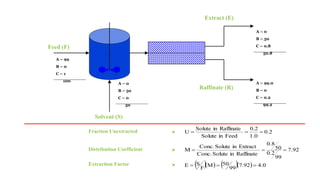
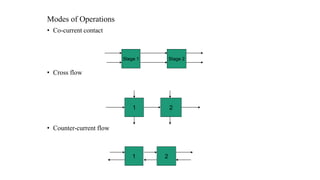
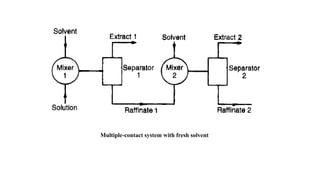
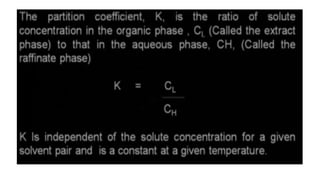
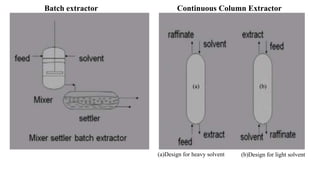
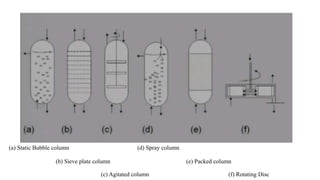
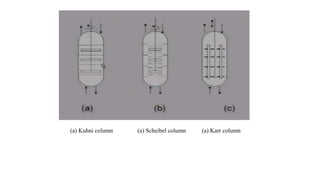
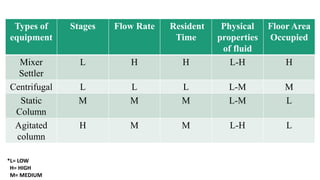
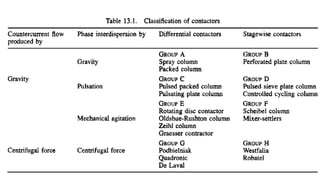
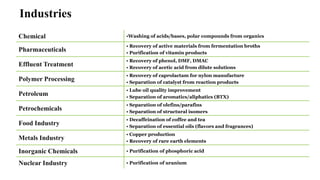
The document details various liquid-liquid extraction techniques and their applications across multiple industries, including pharmaceuticals, petrochemicals, and food processing. It emphasizes the principles and parameters affecting extraction efficiency, as well as the necessary equipment and operational modes for effective separation. Additionally, it compares extraction methods to distillation and provides data on different organic compounds used in these processes.



![Distillation vs. Extraction
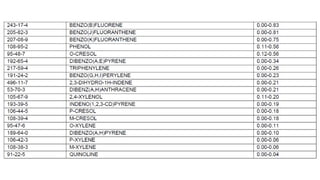
Organic Compound BP [°C]
Water Solu.
[%]
Azeotrope
B.P. [°C]
Azeotrope
Water [%]
Typical Reduction Level
Methylene Chloride 40 2.0 38.1 1.5 < 50 ppb
Acetone 56.2 Infinite Non Azeotropic < 50 ppb
Methanol 64.5 Infinite Non Azeotropic < 50 ppb
Benzene 80.1 0.18 69.4 8.9 < 50 ppb
Toluene 110.8 0.05 85.0 20.2 < 50 ppb
Formaldehyde -21 Infinite Non Azeotropic < 1,000 ppm
Formic Acid 100.8 Infinite 107.1 22.5 < 500 ppm
Acetic Acid 118.0 Infinite Non Azeotropic < 500 ppm
Pyridine 115.5 57 92.6 43 < 10 ppm
Aniline 181.4 3.60 99.0 80.8 < 10 ppm
Phenol 181.4 8.20 99.5 90.8 < 10 ppm
Nitrobenzene 210.9 0.04 98.6 88.0 < 10 ppm
Dinitrotoluene (2,4) 300.0 0.03 99 – 100 > 90 < 10 ppm
Dimethyl Formamide 153.0 Infinite Non Azeotropic < 10 ppm
Dimethyl Acetamide 166.1 Infinite Non Azeotropic < 10 ppm
n-Methylpyrrolidone 202.0 Infinite Non Azeotropic < 10 ppm
ExtractionDistillation](https://image.slidesharecdn.com/liquid-liquidextraction-190205091504/85/Liquid-liquid-extraction-6-320.jpg)