This document discusses antibiotic sensitivity testing and provides information on:
1. Common antibiotic classes and mechanisms of resistance.
2. Methods for antibiotic sensitivity testing including disk diffusion, E test, and broth dilution.
3. Important resistant bacteria like MRSA, VRE, ESBLs, CREs, and guidelines for detecting them.
4. Interpreting results and quality control for antibiotic sensitivity testing.

















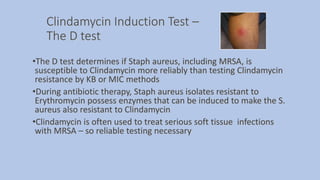

![Extended Spectrum Beta Lactamase [ESBL]
•Enzymes produced by Enteric Gram negative bacilli
• Confer resistance to Cephalosporins, Penicillins and Monobactam
(Aztreonam) antibiotics by opening the beta lactam ring of the antibiotic
and inactivating the antibiotic
• ESBLs do not attack Cephamycin (cefoxitin, cefotetan) or the
Carbapenem antibiotic classes
•Plasmid mediated CTX-M beta lactamases are the most common ESBL
enzymes in the US currently, but many more ESBL types can be found
worldwide
•Therapy for ESBL producing gram negative rods:
• Carbapenems: Imipenem, Meropenem, Doripenem, and
Ertapenem](https://image.slidesharecdn.com/webpagesusceptiblity2020-200327042329/85/Antibiotic-Sensitivity-Testing-2020-Update-21-320.jpg)





