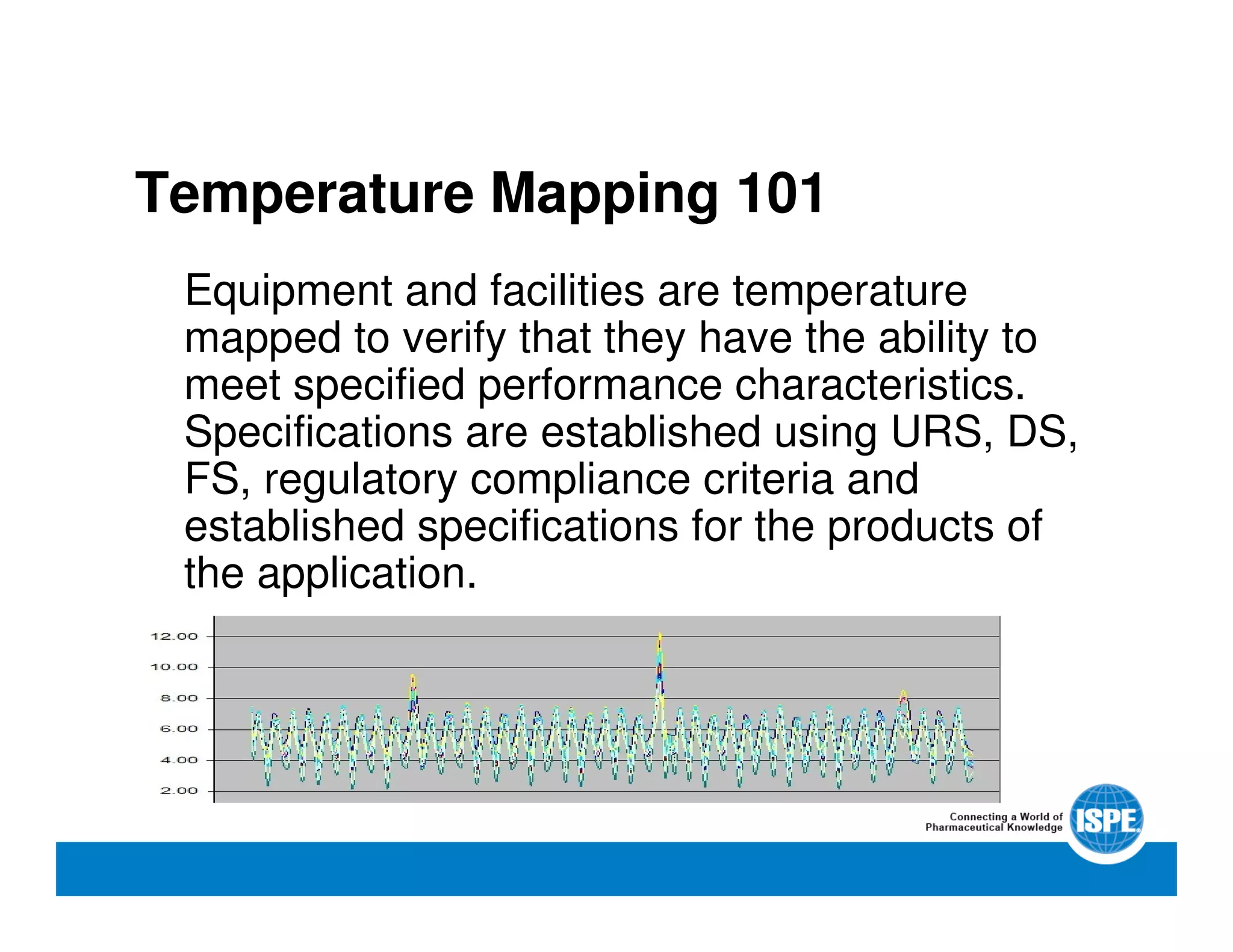
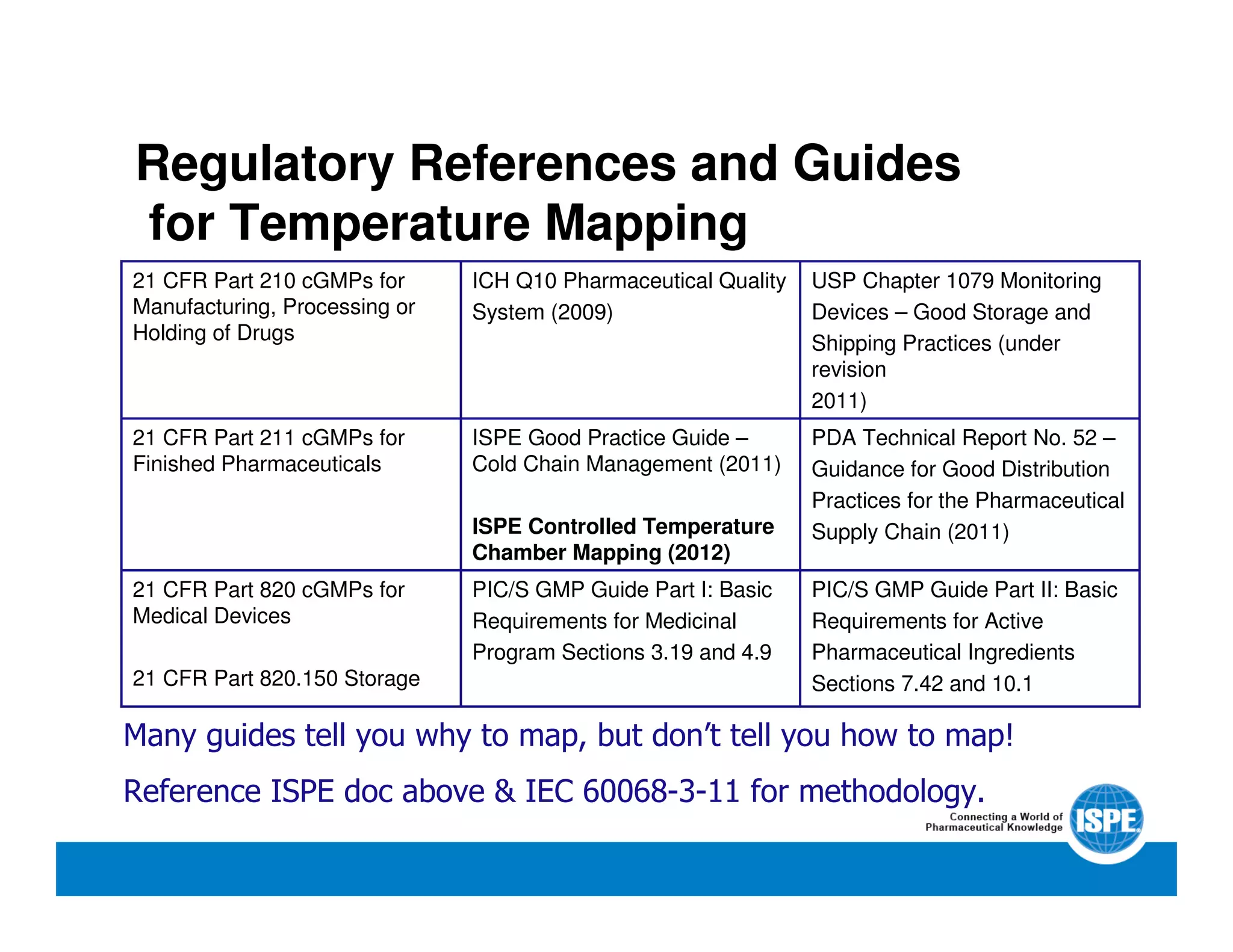
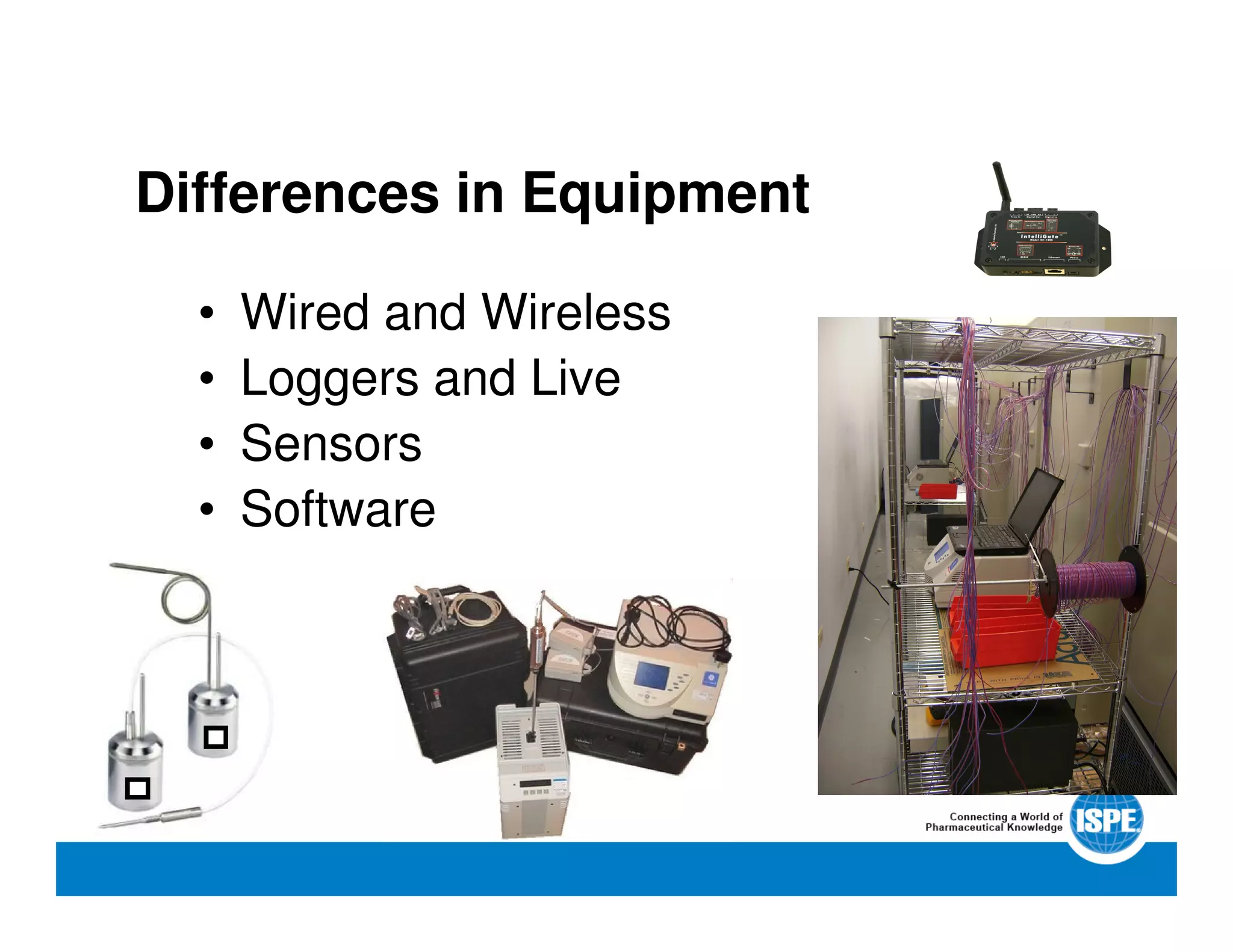
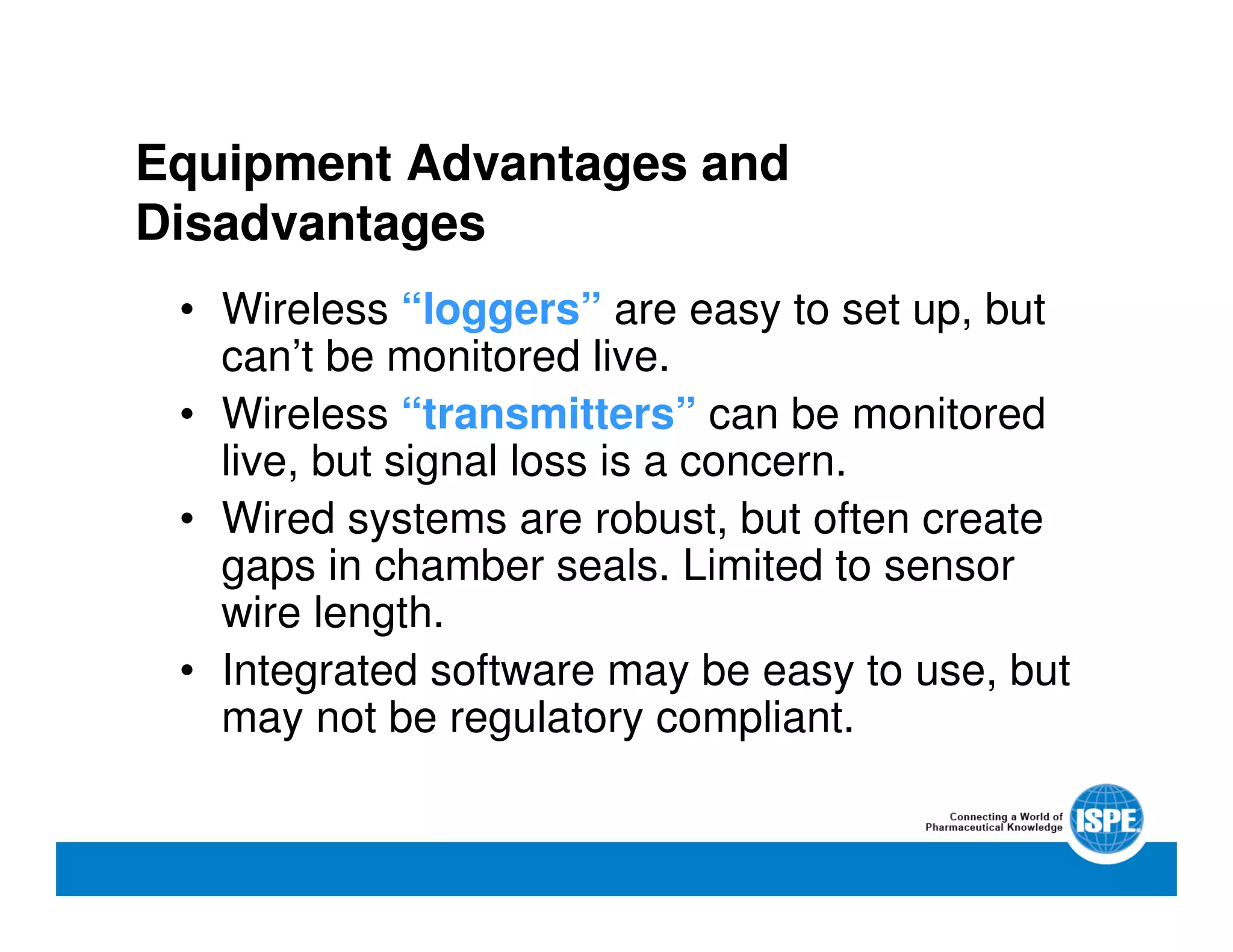
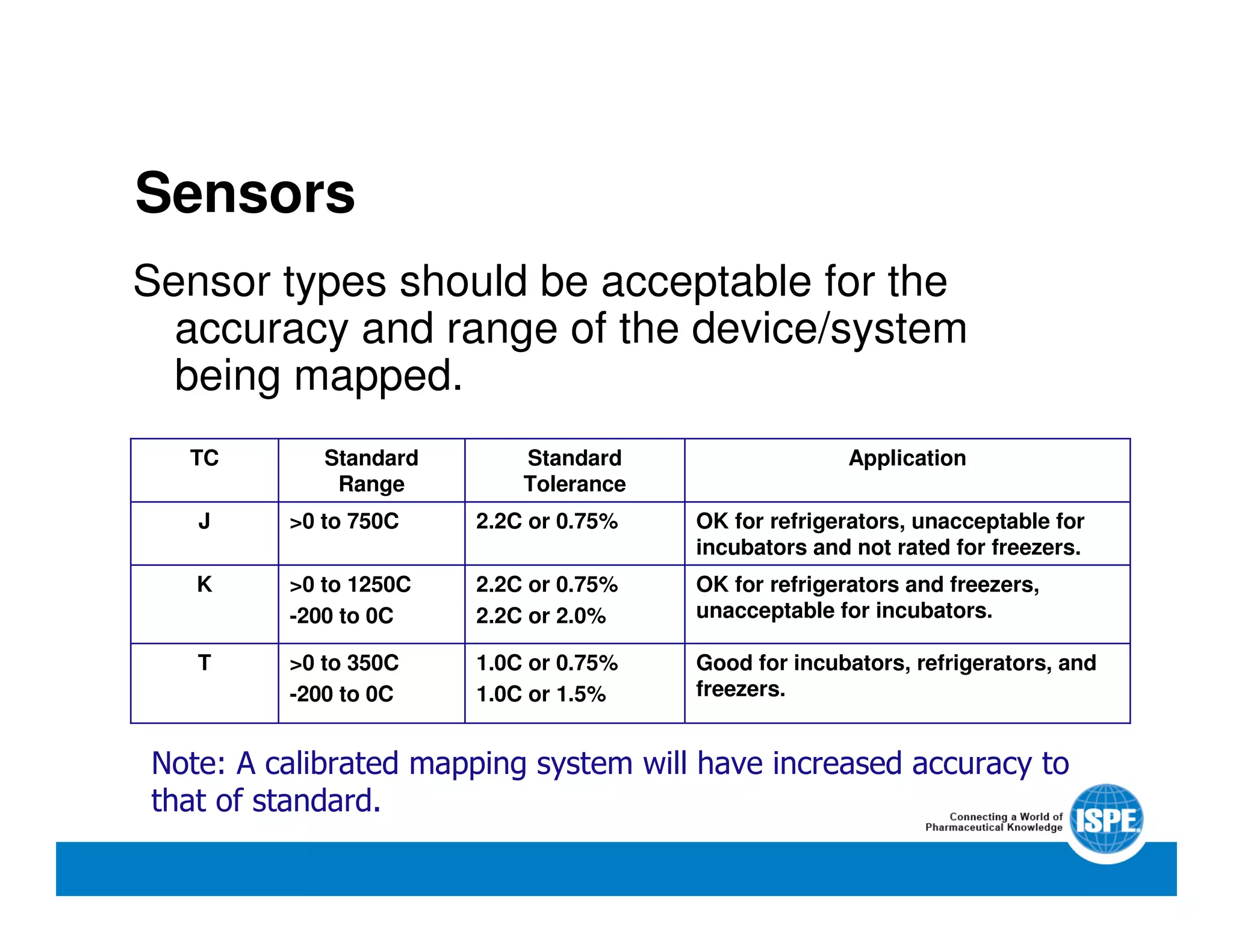
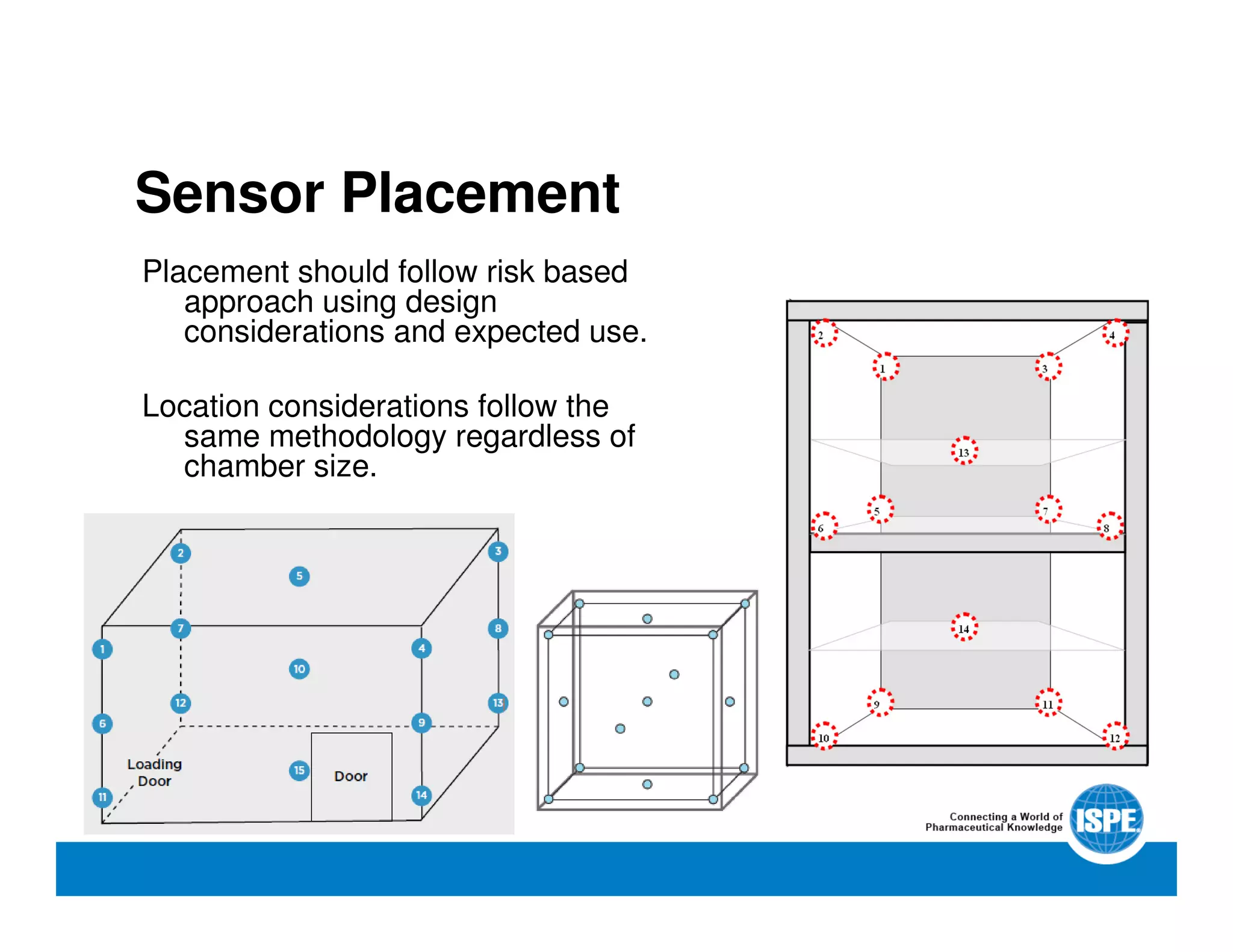
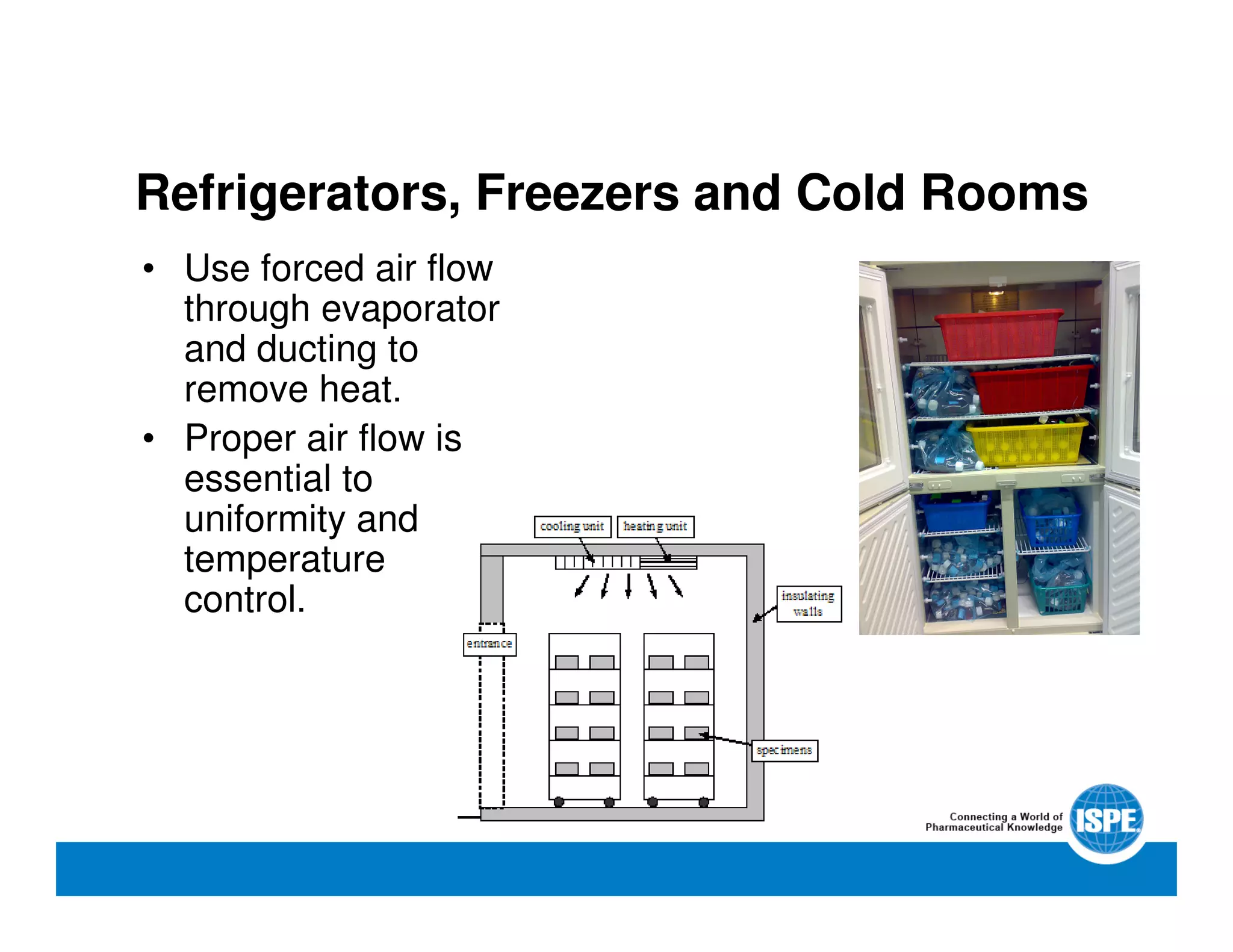
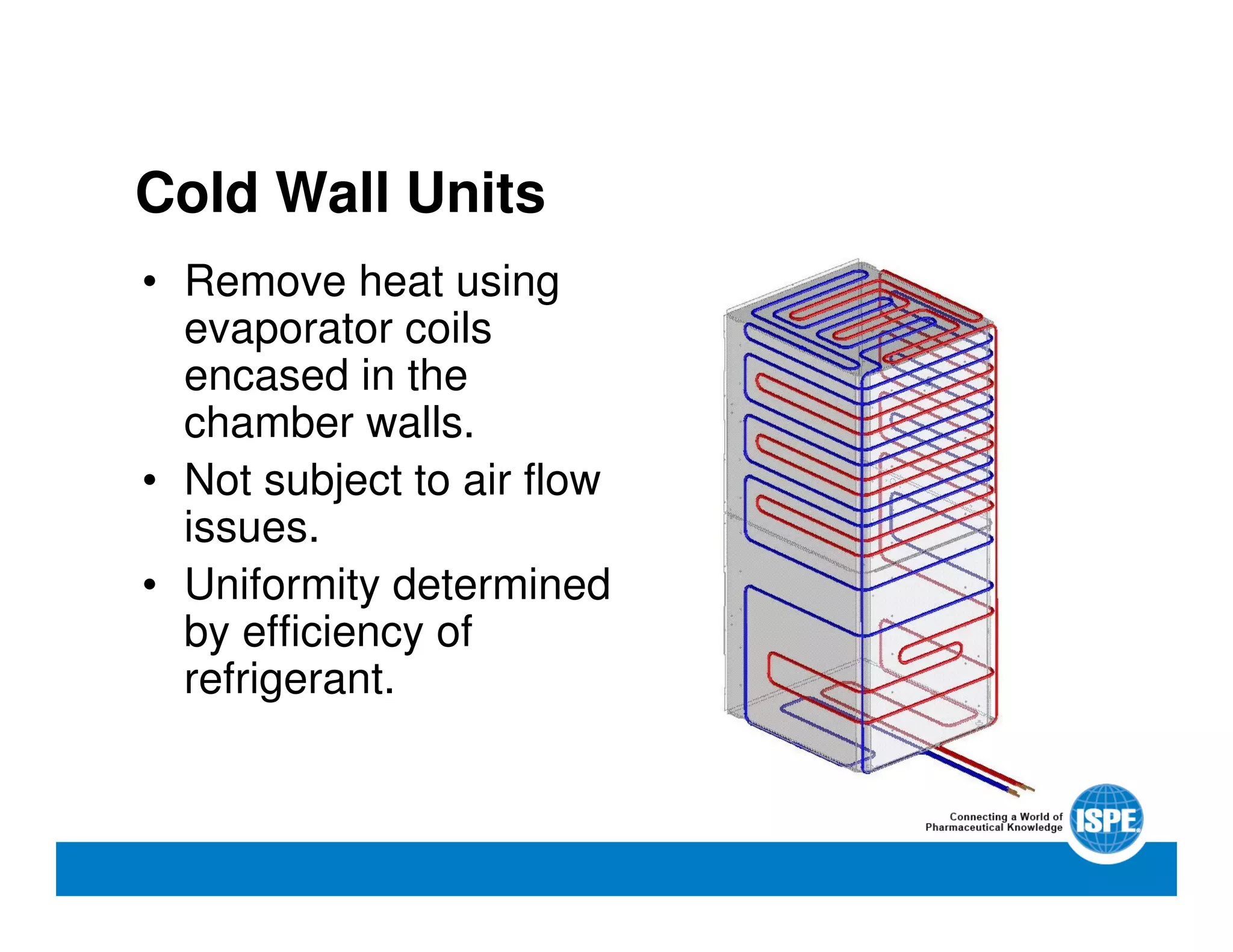
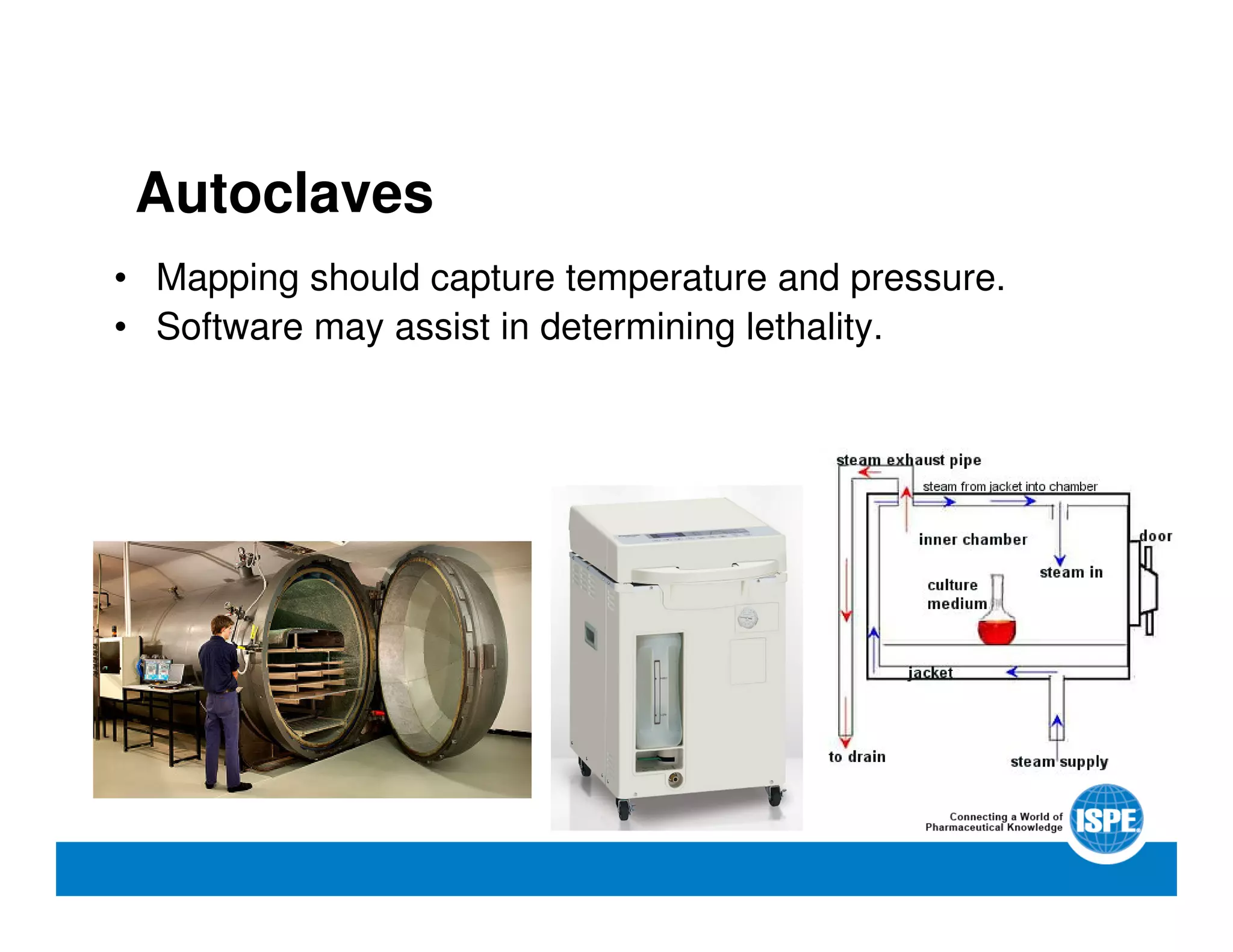
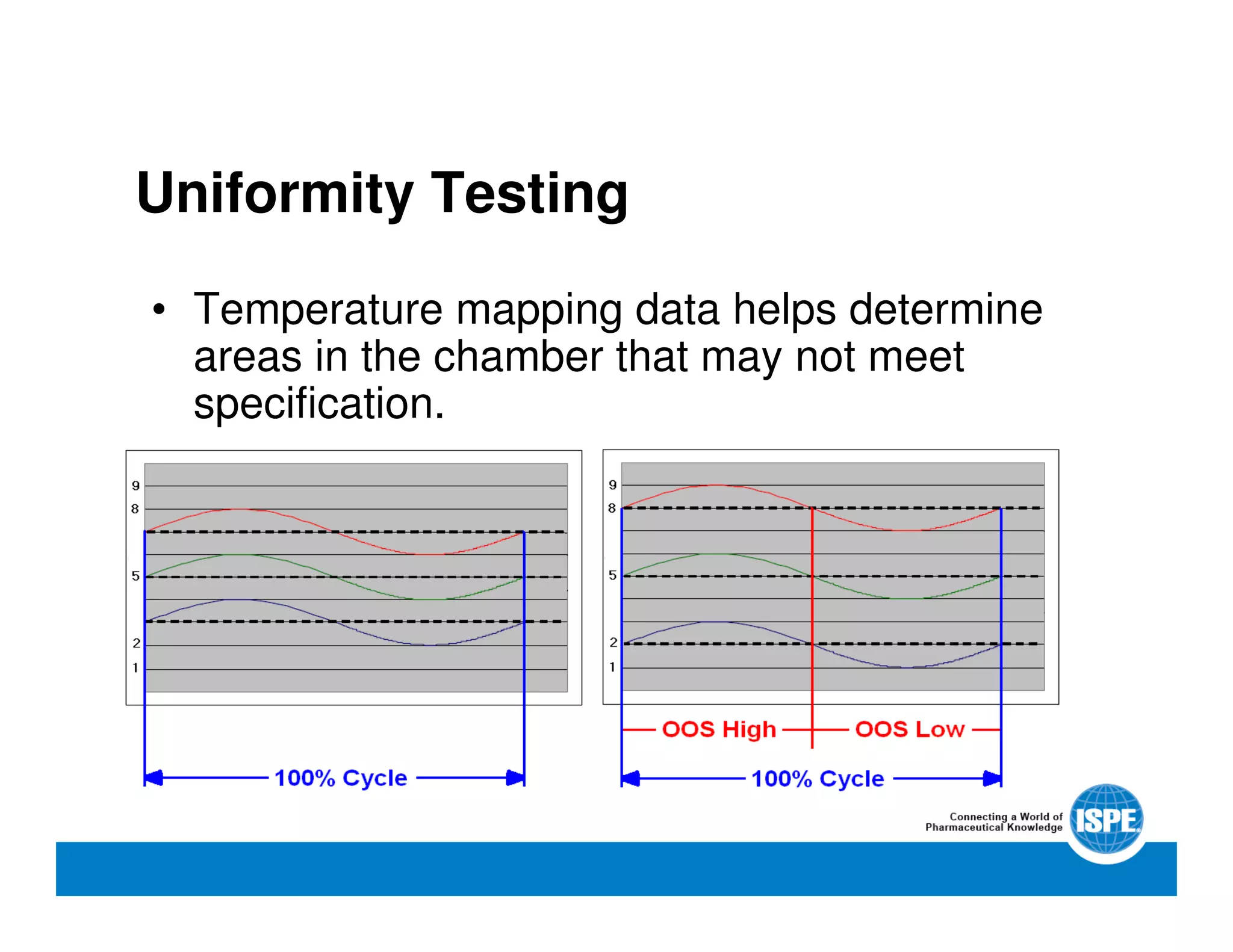
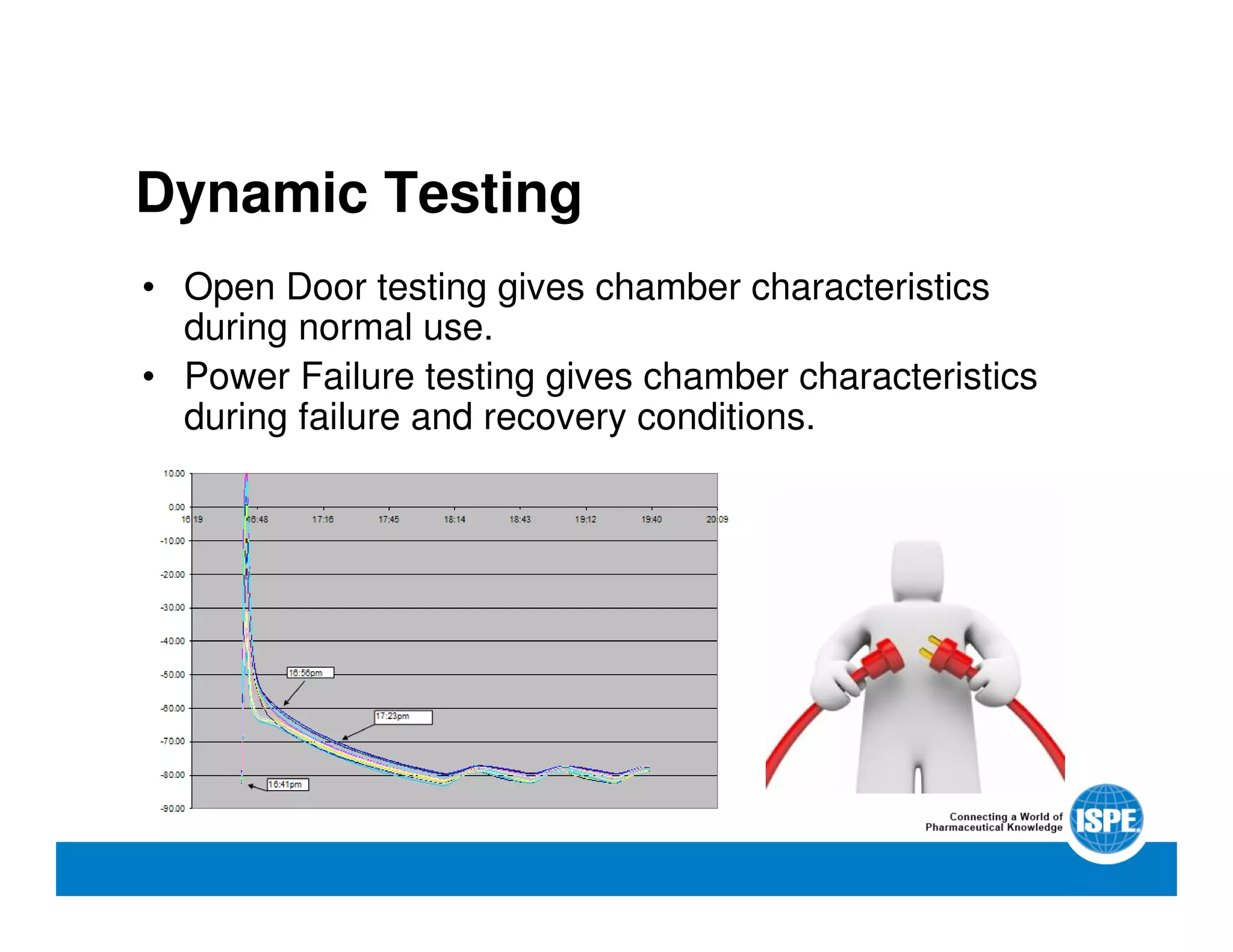
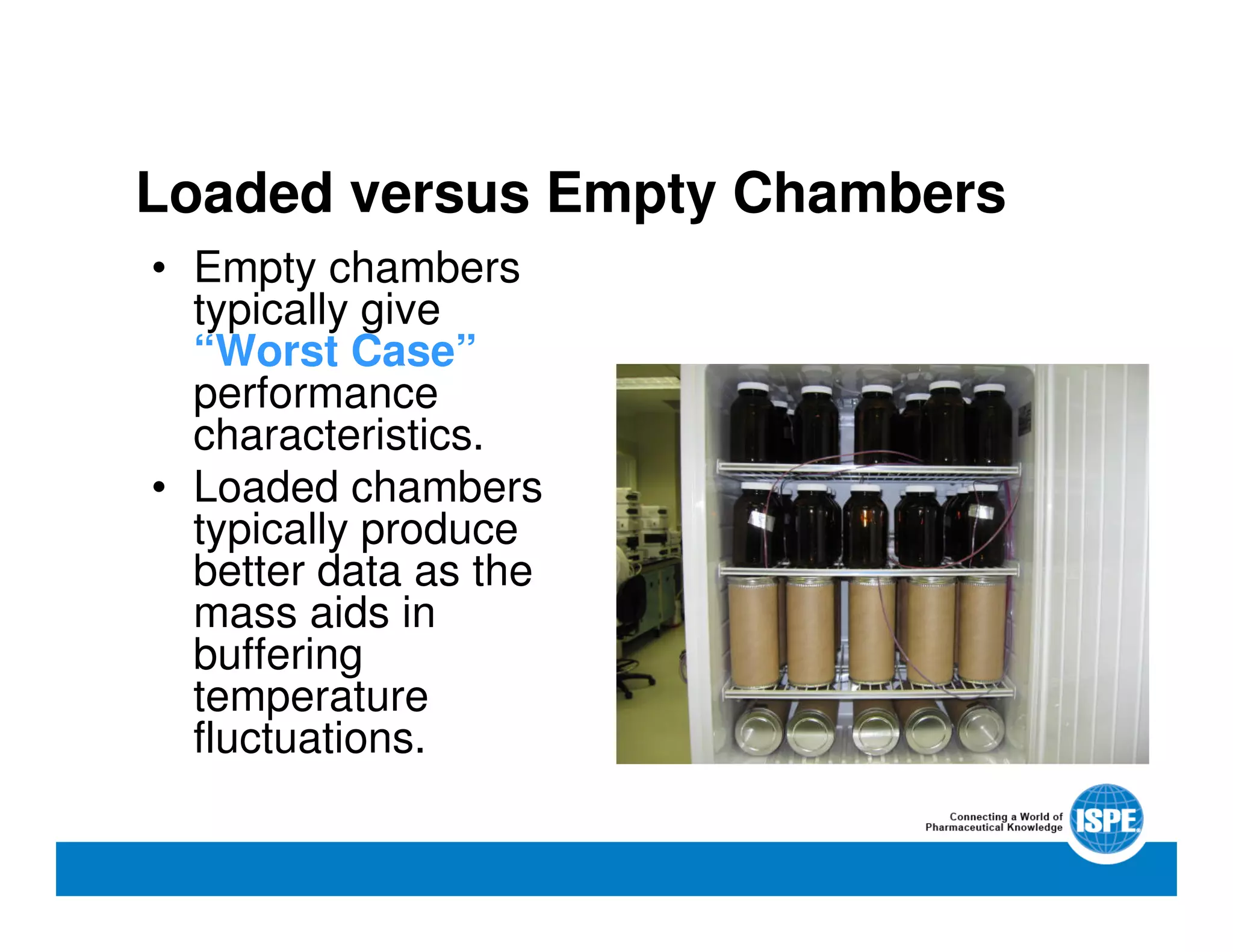
The document outlines the procedures and equipment necessary for temperature mapping in healthcare, emphasizing compliance with regulatory standards. It details the steps for effective temperature mapping, including critical mapping points, data logger criteria, and sensor types, alongside the advantages and disadvantages of various equipment. Additionally, it highlights the importance of air flow and proper placement in different storage units and chambers to ensure temperature uniformity.