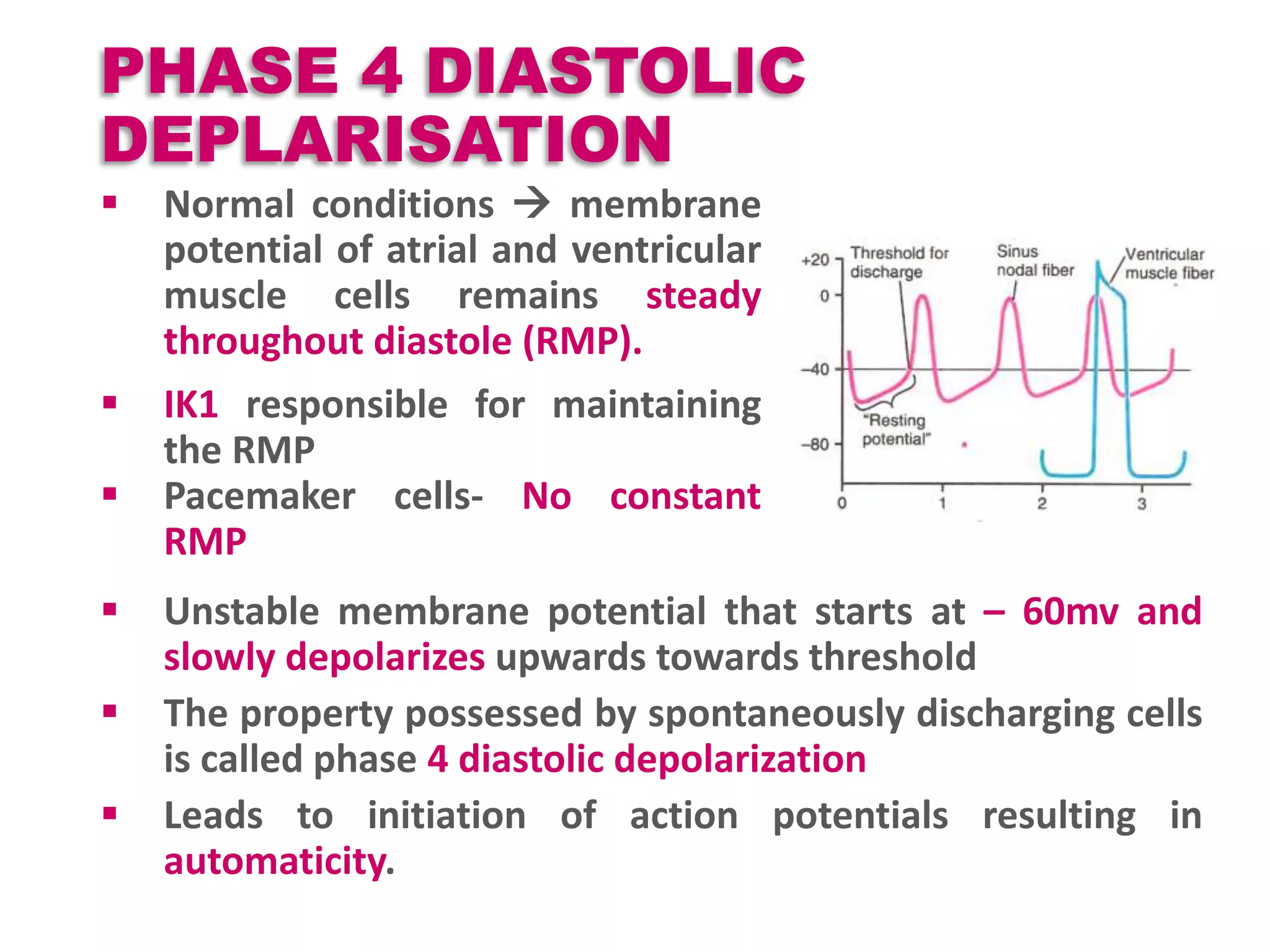
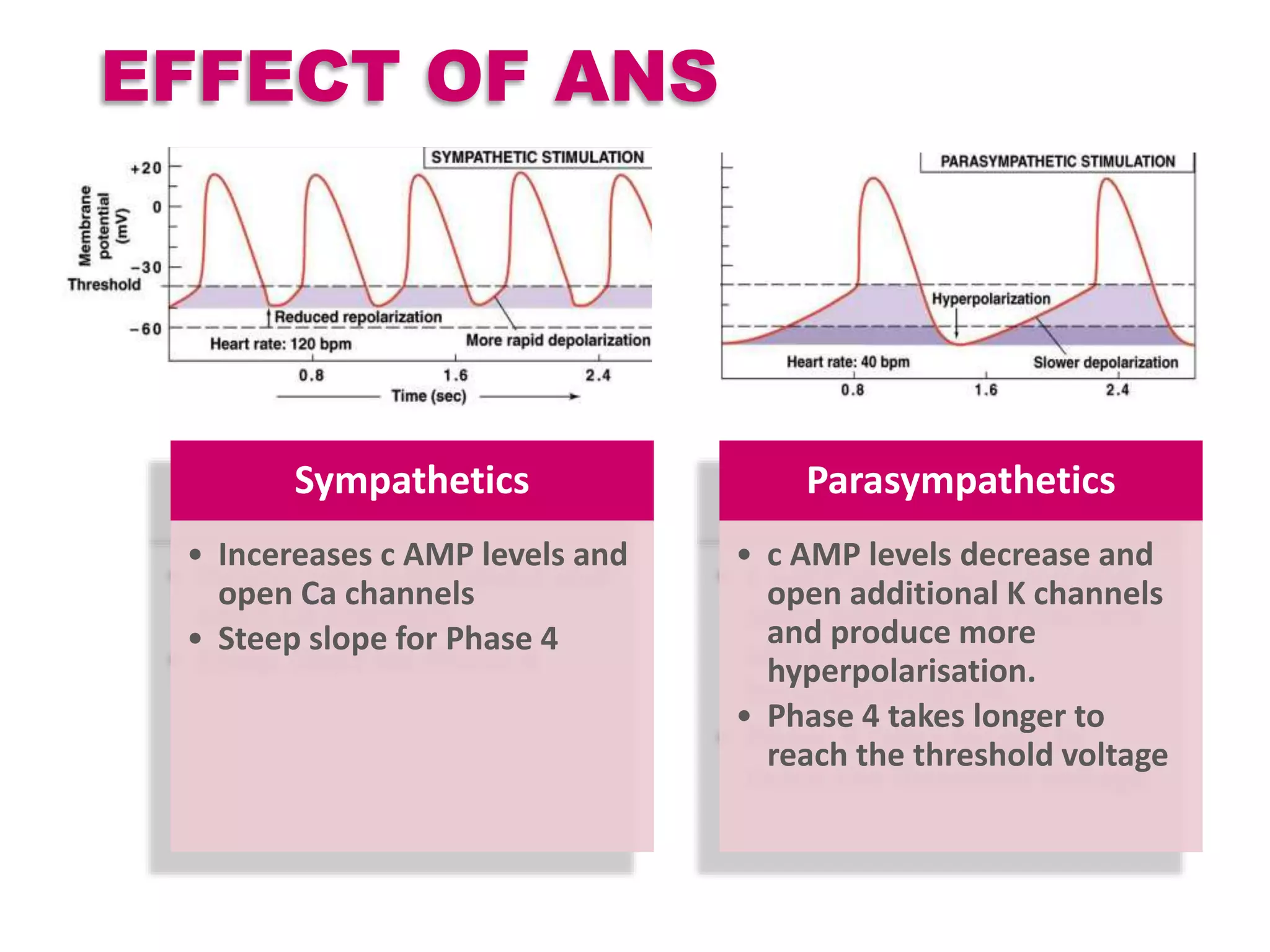
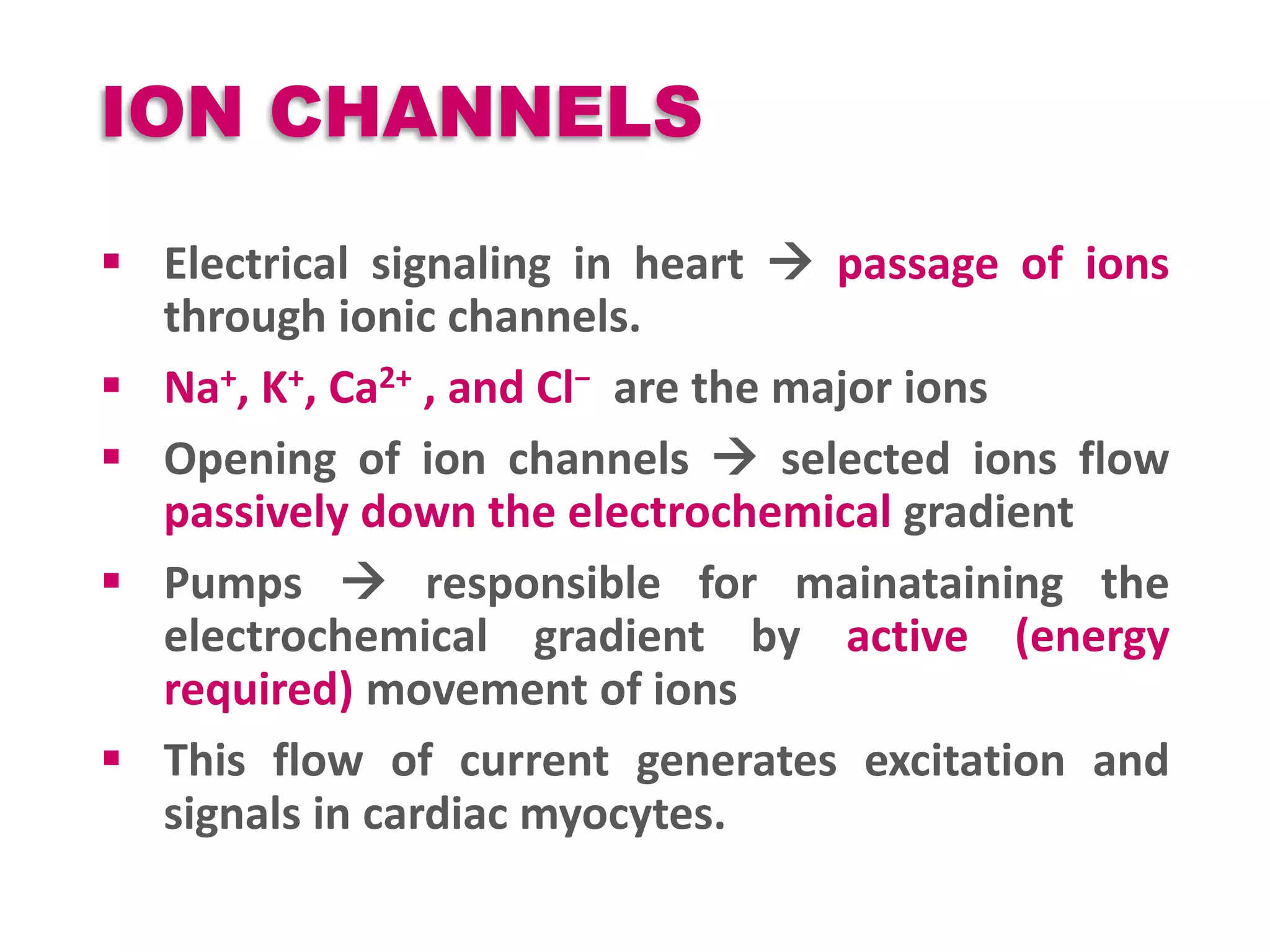
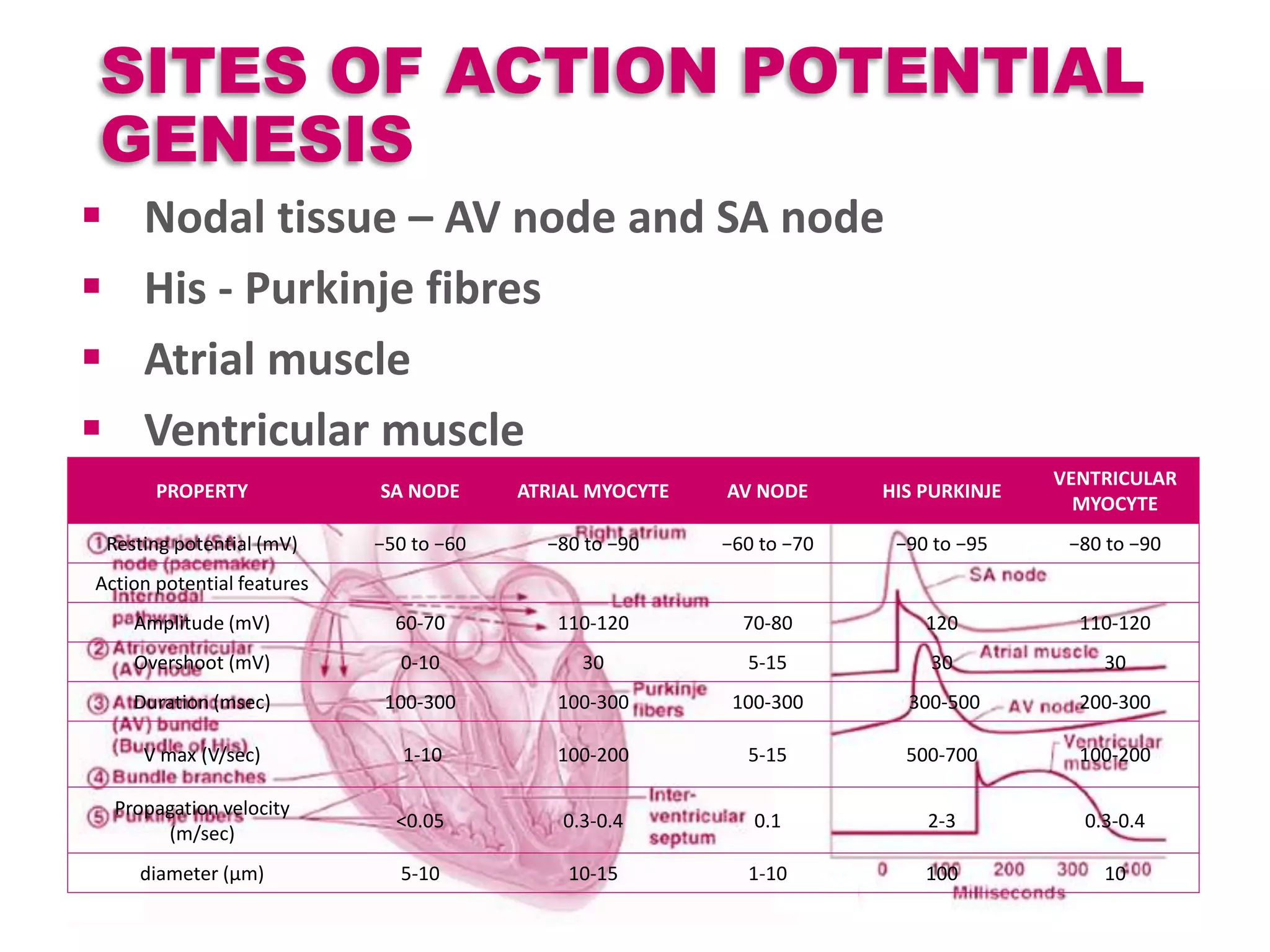
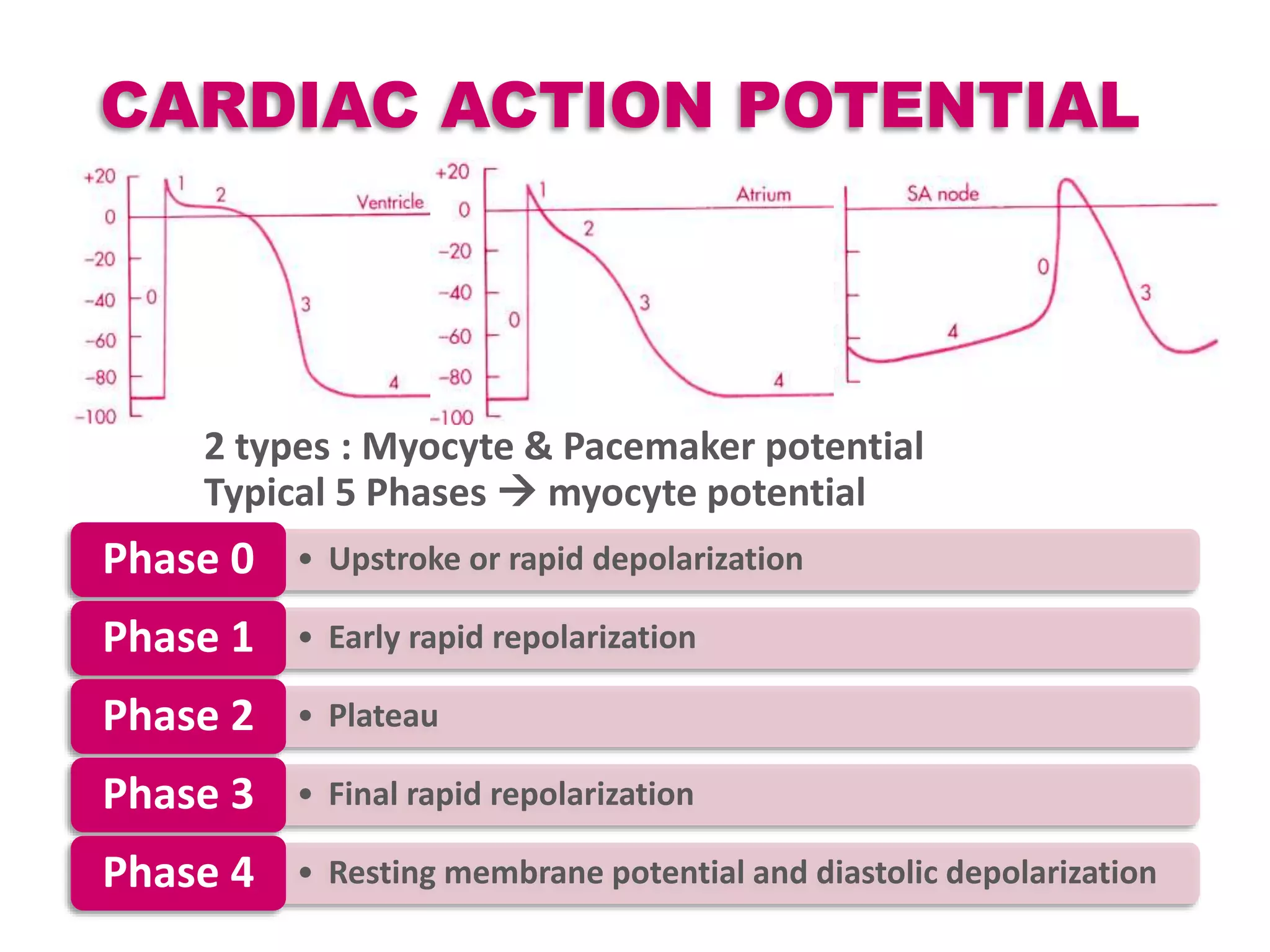
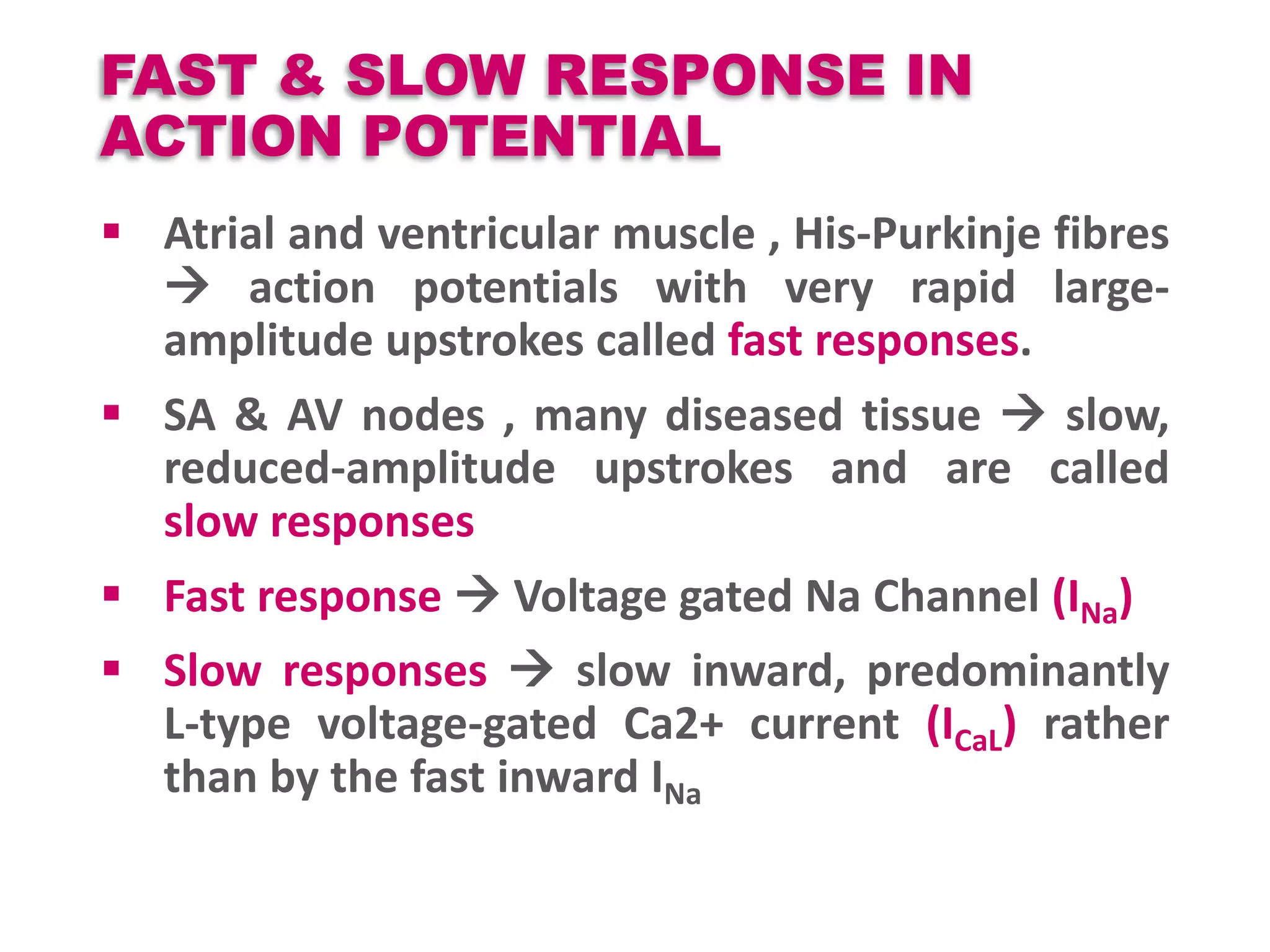
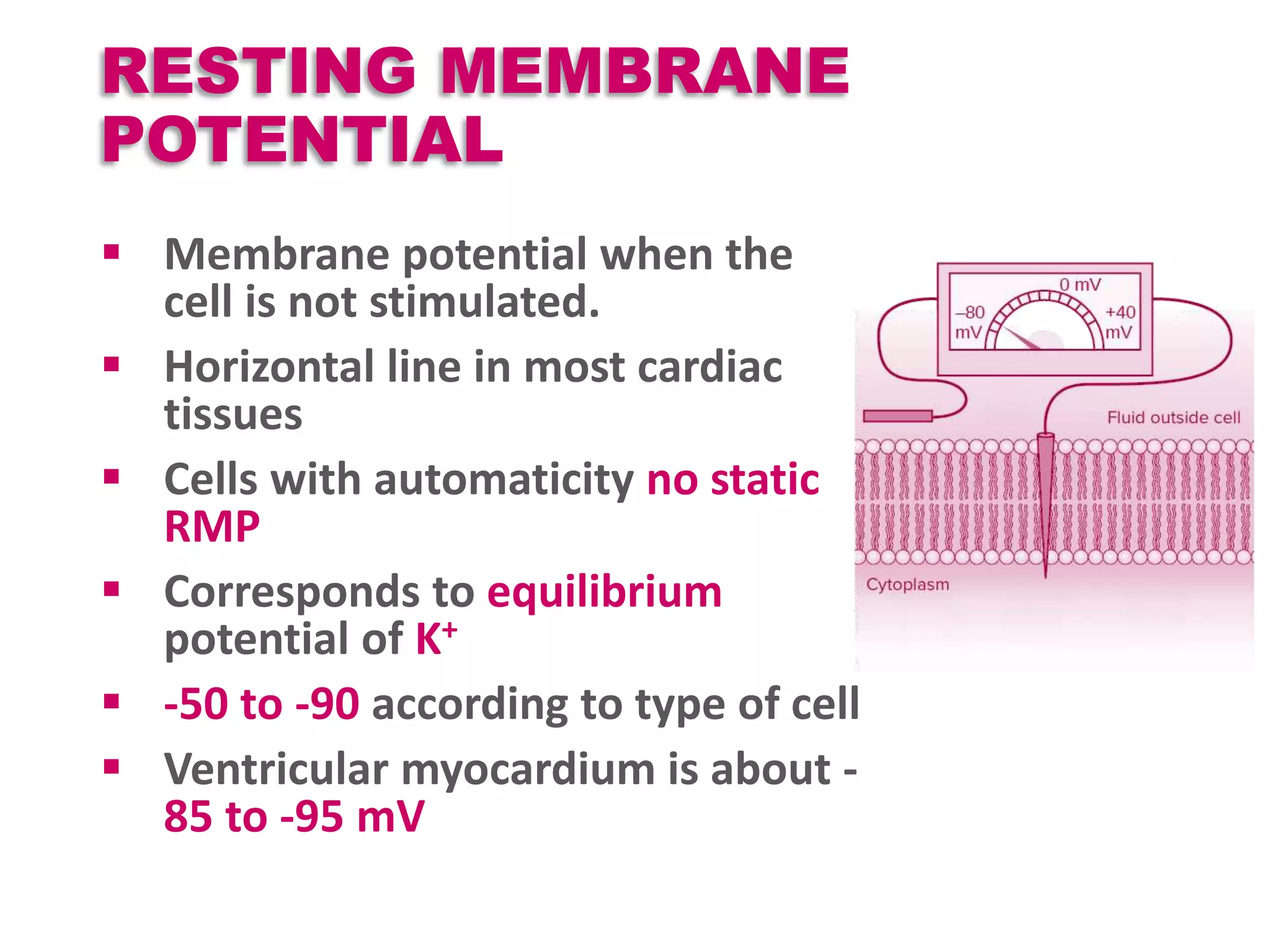
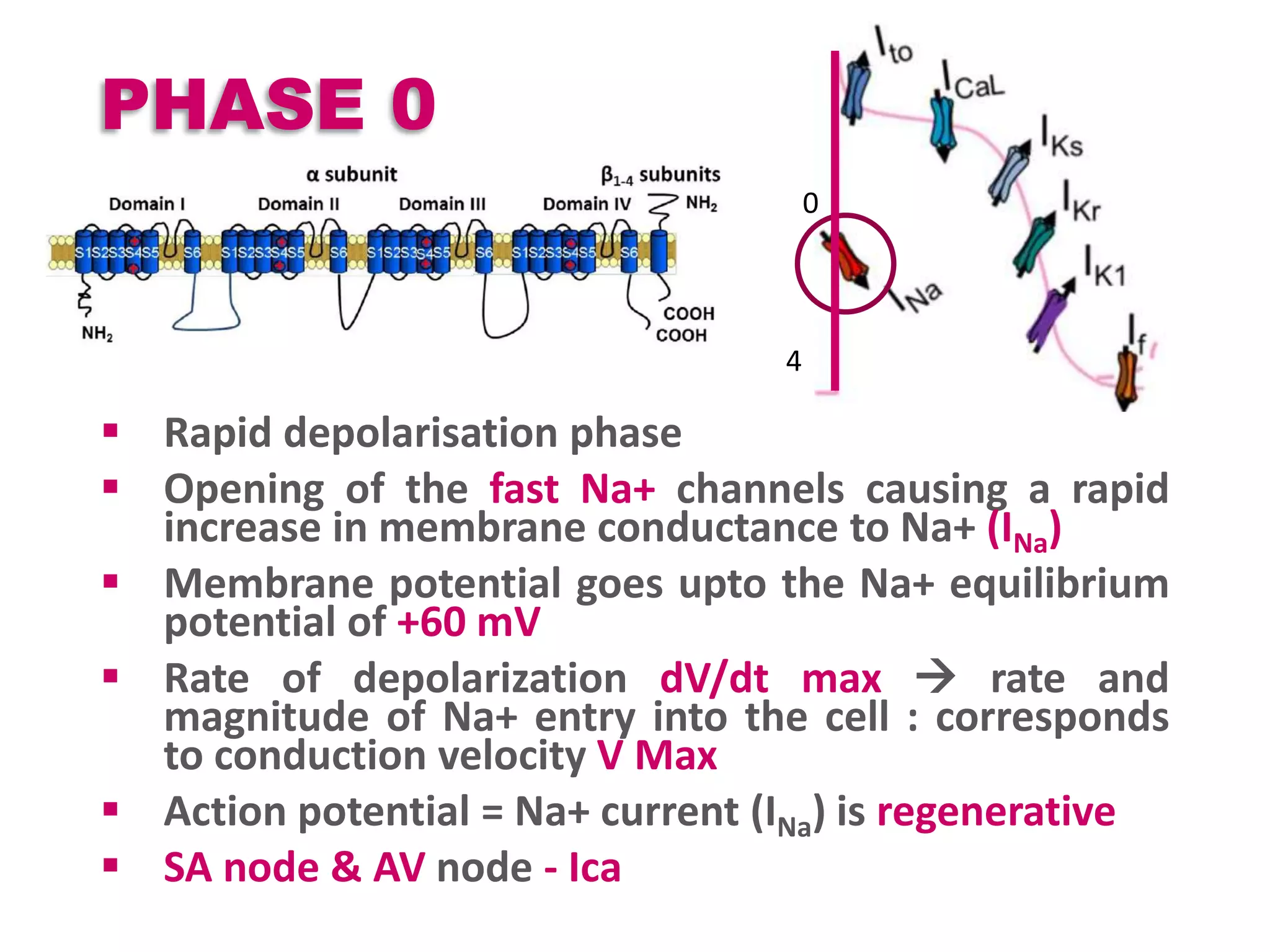
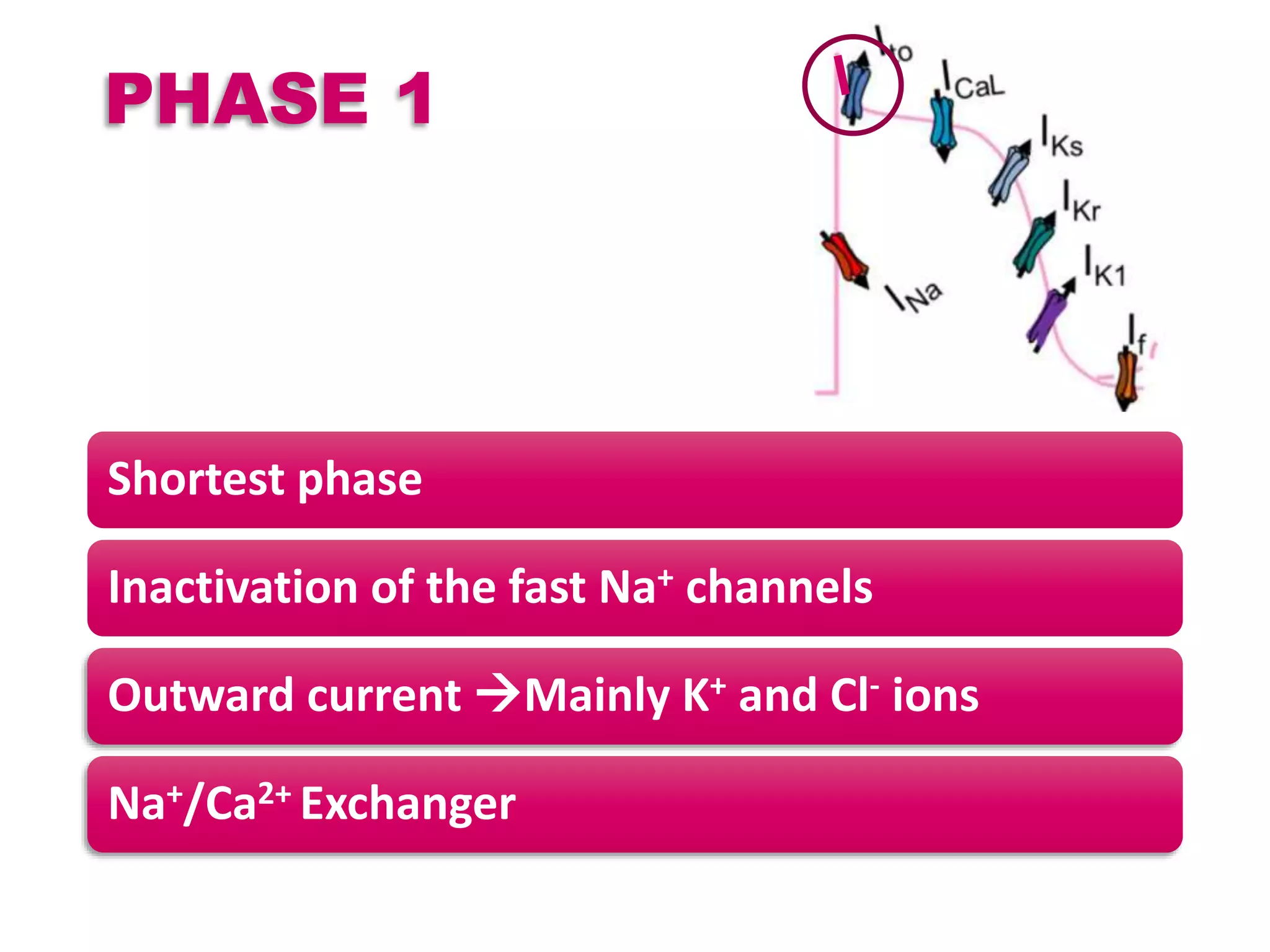
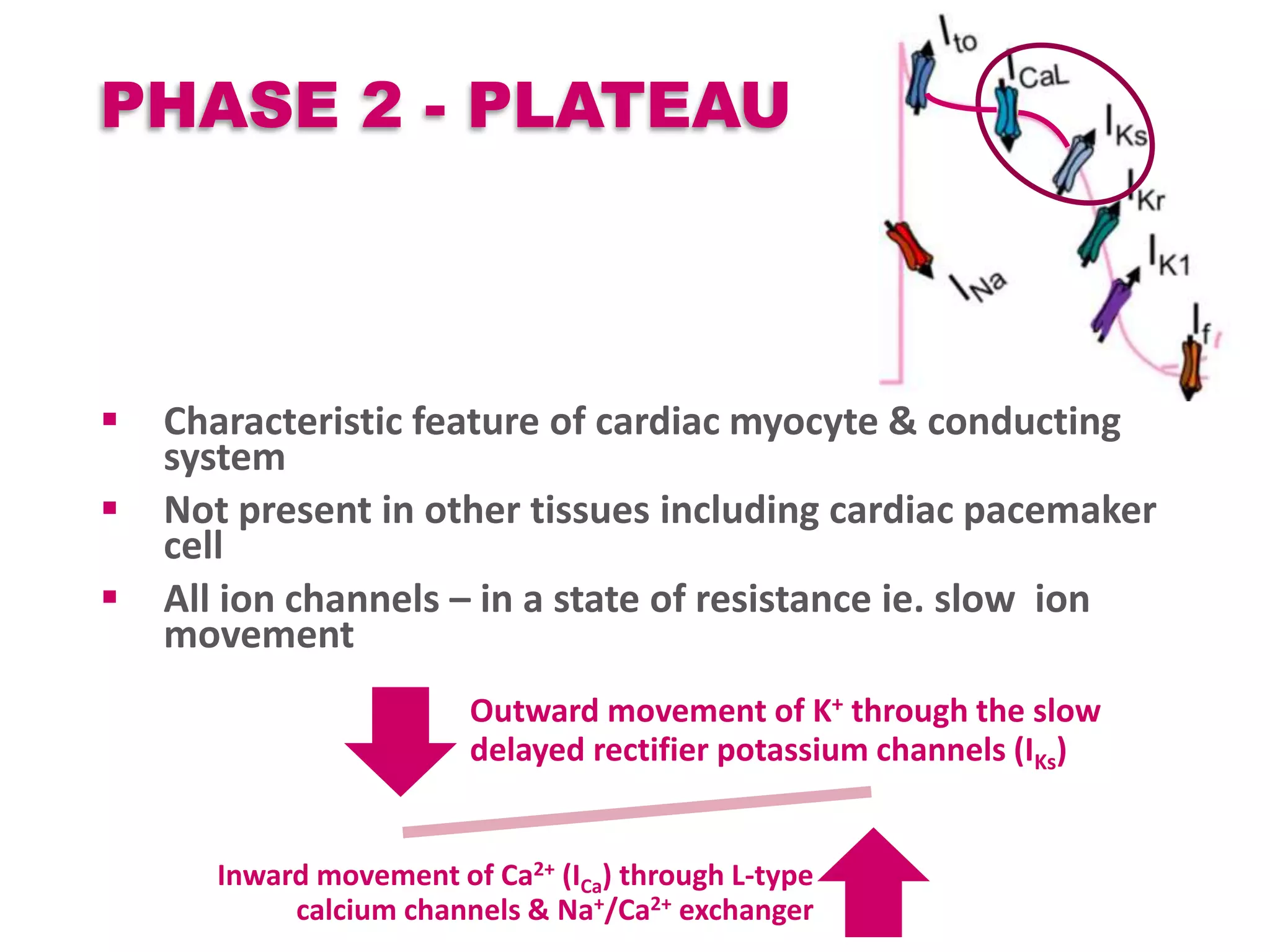
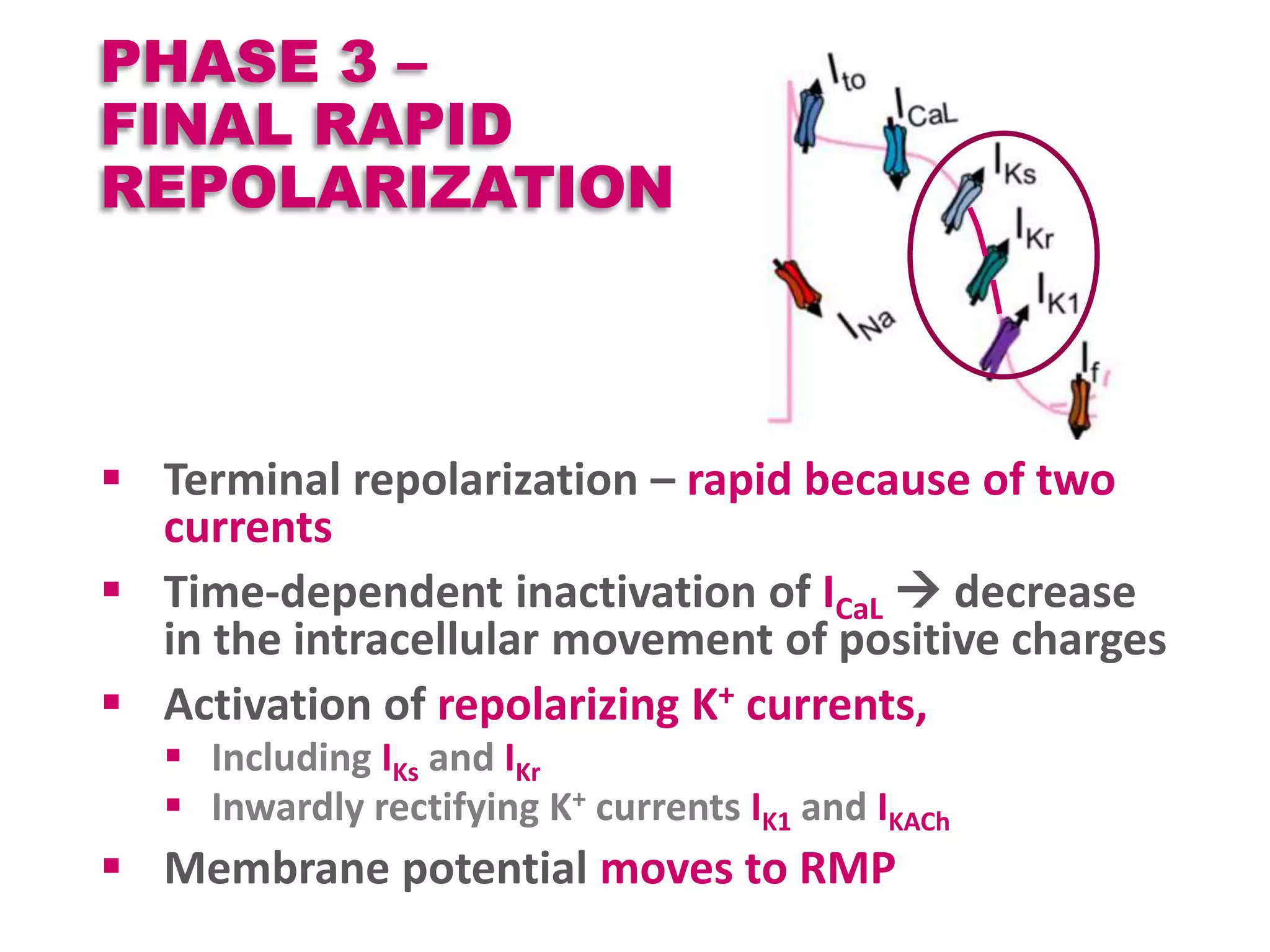
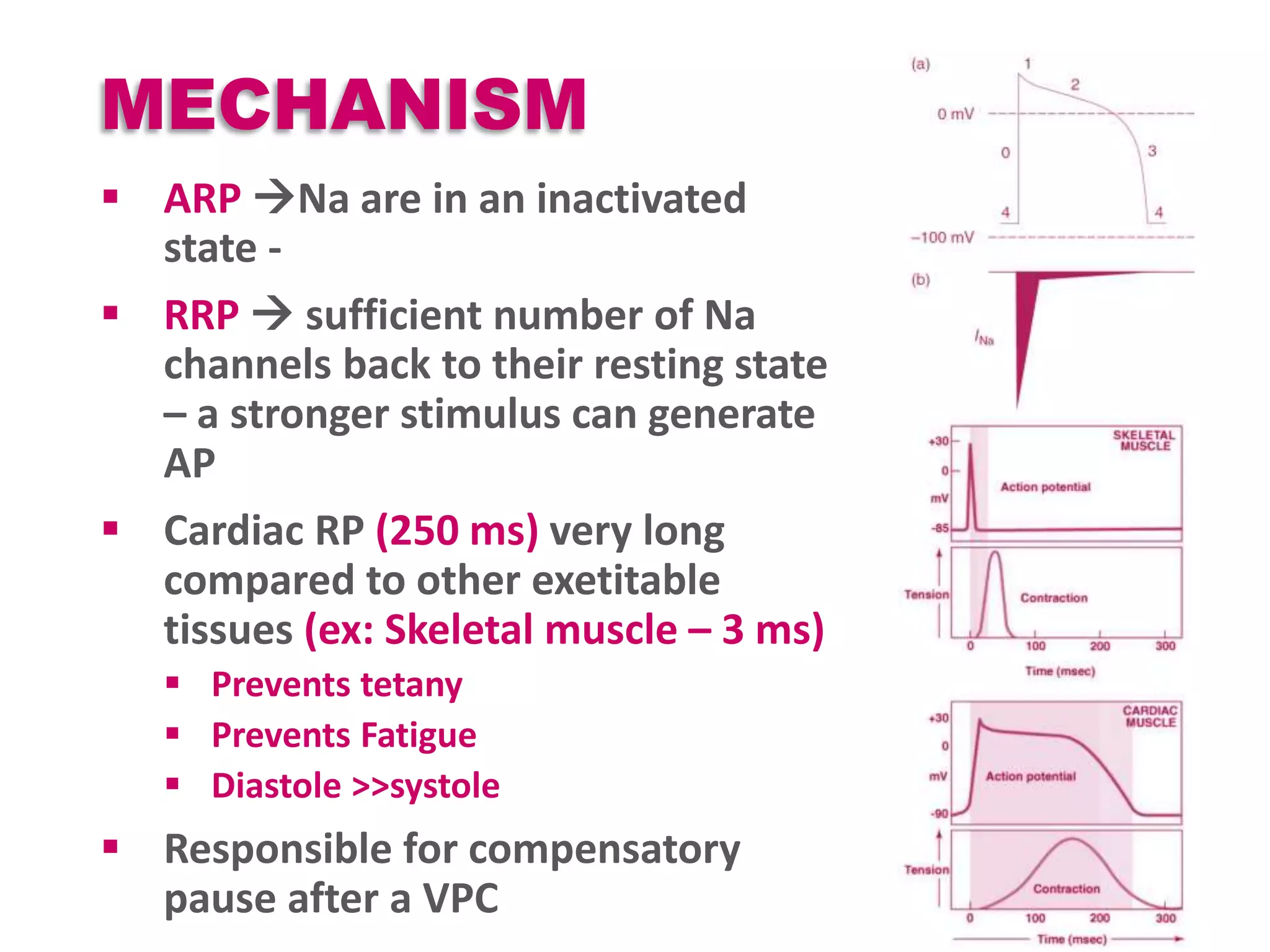
Cardiac action potentials arise from the coordinated movement of ions through membrane channels in cardiac cells. The cardiac action potential has 5 phases: rapid upstroke (phase 0) due to sodium influx, early rapid repolarization (phase 1) mediated by potassium currents, plateau phase (phase 2) maintained by calcium and potassium currents, final rapid repolarization (phase 3) due to potassium currents, and resting phase (phase 4) where the cell prepares for the next action potential. Precisely regulated ion channel function underlies the generation and propagation of action potentials and ensures normal cardiac rhythm.

![EQUILIBRIUM POTENTIAL
Also called the, reversal, or Nernst potential of the
channel.
The potential at which the passive flux of ions resulting
from the chemical driving force is exactly balanced by the
electrical driving force is Given by the Nernst equation
Es =RT/ZF ln [S]2 / [S]1
T is temperature [kelvin] , R is the gas constant , F is the Faraday constant
Z is the valence of ion
[S]2 and [S]1 are the final concentrations of the ion in outer & inner
compartments
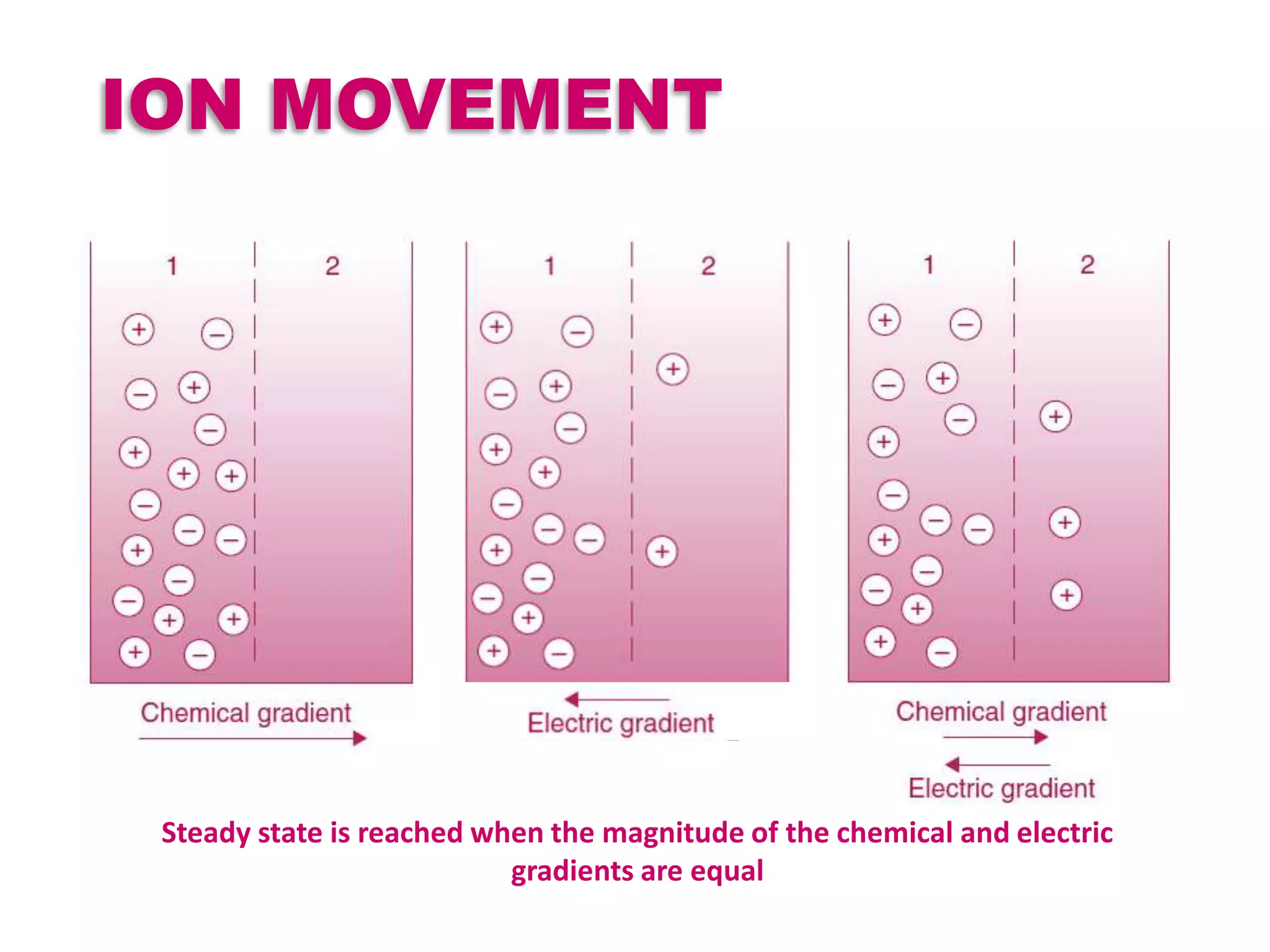
At equilibrium potential net diffusion is 0
All ions try to reach equilibrium i.e., tries to drive the
membrane potential towards its equilibrium potential
At RMP, membrane is permeable mostly to potassium ,
hence RMP is close to the EK](https://image.slidesharecdn.com/cap2recovered-190324101030/75/Cardiac-action-potential-4-2048.jpg)





![ACTION POTENTIAL
Sudden rise & fall in membrane voltage
in a characteristic pattern
Depolarization followed by
repolarization
Passive movement of ions across electro
chemical gradient established by active
ion pumps
Net current of all open channels [amplitude & direction]
Depends on 2 factors
Electromechanical gradient across
Open Channels
Fixed time & voltage relationship according to the specific cell type
Neurons few milliseconds , Cardiac fibers - several 100 milliseconds](https://image.slidesharecdn.com/cap2recovered-190324101030/75/Cardiac-action-potential-11-2048.jpg)


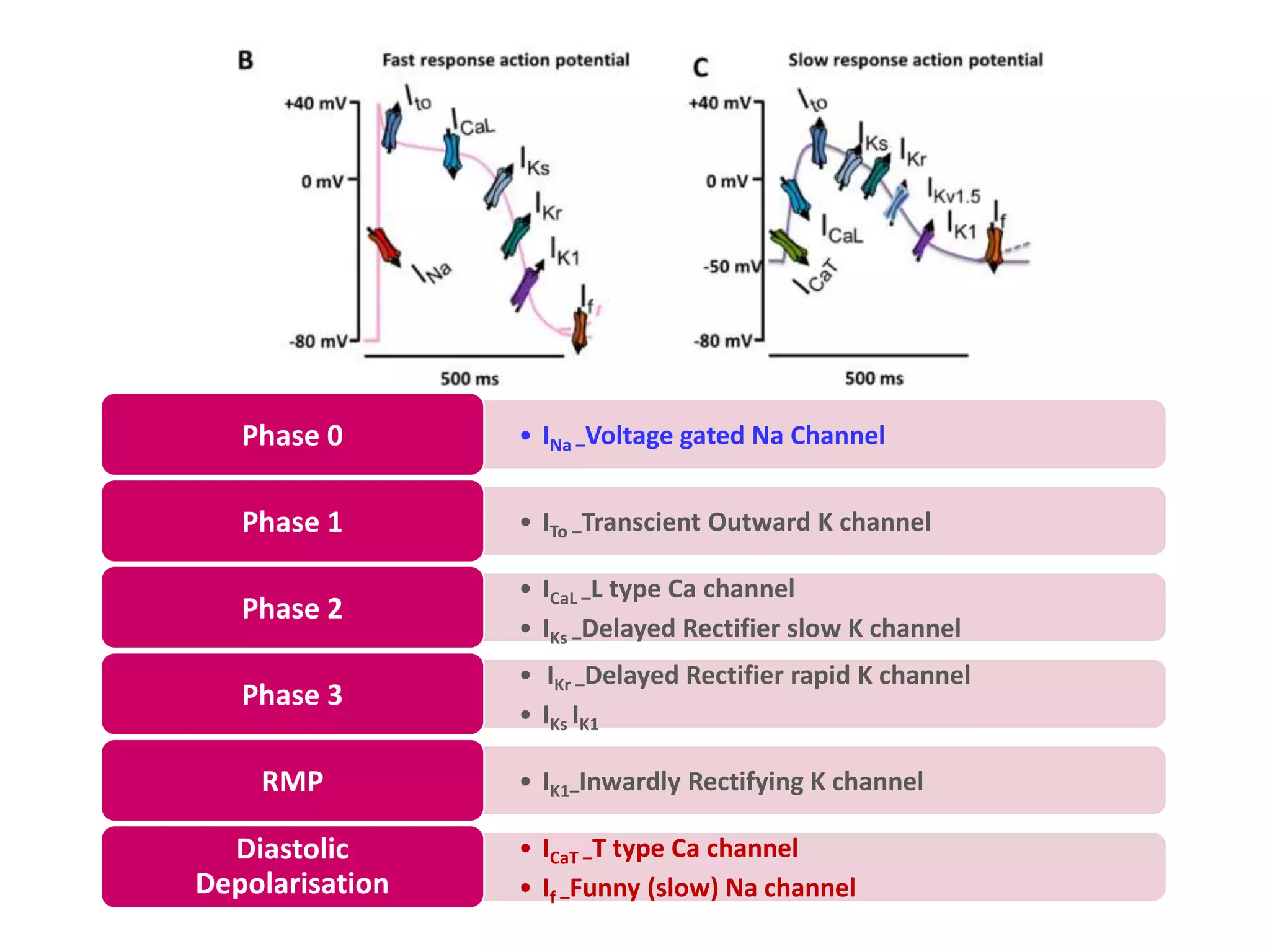
![IONS - RMP
Outward K+ current – Inwardly rectifying K+
channels(IK1 ) -contributes to RMP atrial and
ventricular myocytes, as well as in Purkinje cells.
Low [K] Voltage leads to less IK1 activity RMP
less negative more excitability
Ca- No direct effect but intra cellular Ca can
influence other ions ex: Cl- & Na+/Ca++ exchanger
Na+ K+ ATPase – 3 Na+ outward and 2 K+ inward
against their gradient.
K+](https://image.slidesharecdn.com/cap2recovered-190324101030/75/Cardiac-action-potential-19-2048.jpg)



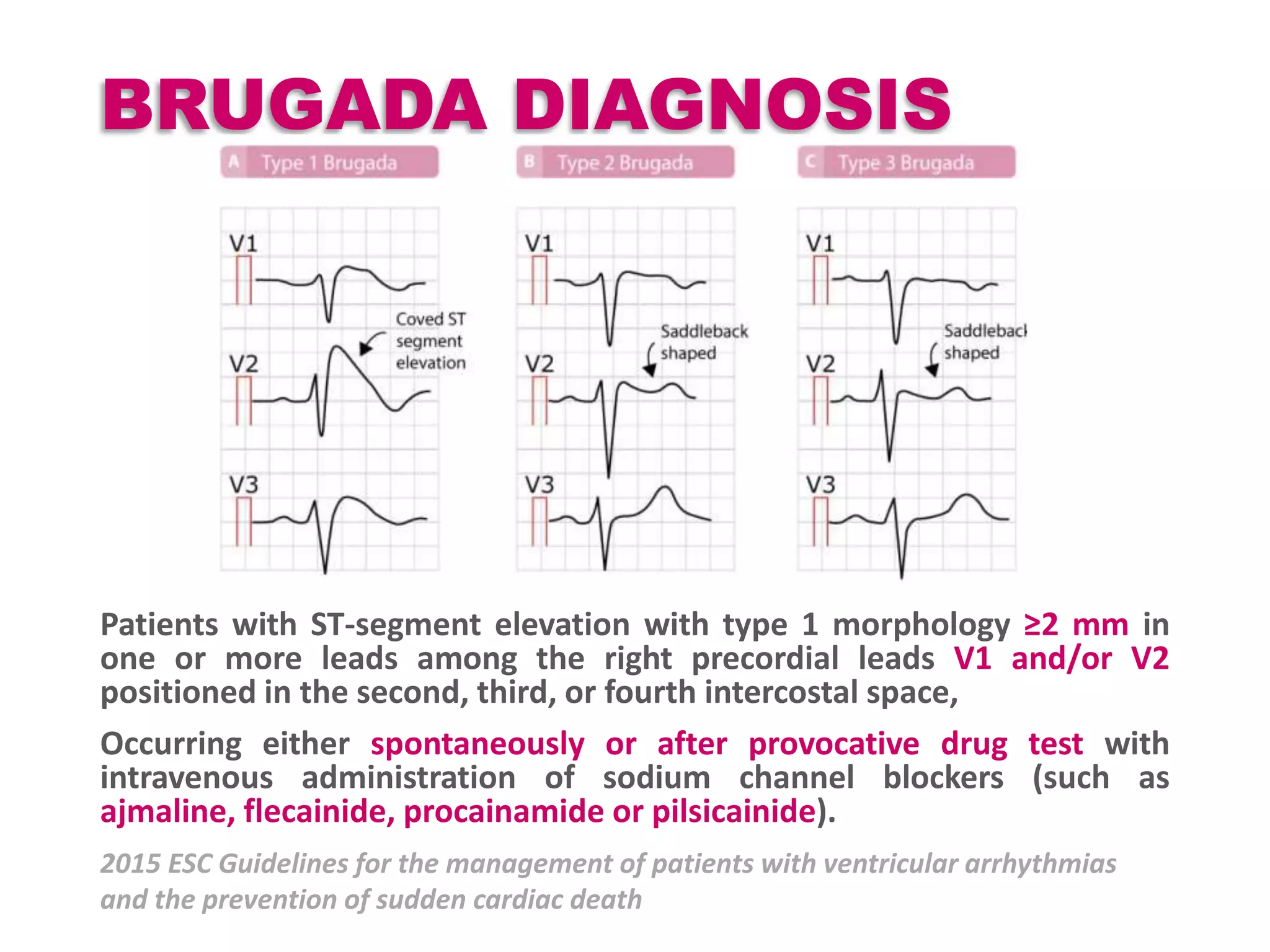
![BRUGADA SYNDROME
Most common AD Loss Of Function SCN5A gene
(encodes for Voltage gated Na channel)
Polymorphic ventricular tachycardias & SCDs
Incidence 1-5 / 10 000 ( High in SEAR)
Na channel blockers [Ajmaline Flecainide and
Procainamide ] used for provocative testing.
Type 2 & 3 patterns should change to Type 1 pattern
with drug testing](https://image.slidesharecdn.com/cap2recovered-190324101030/75/Cardiac-action-potential-24-2048.jpg)


![PHASE 2
Even though gradient of K+ is high after a
depolarization the movement is restricted.
K1 current inwardly rectified
Delayed rectifier K current Rapid (IKr) &
slow(IKs)
Rapid channels inactivated by the depolarization
current.
This fast inactivation sensitive to extracellular
[K+] Accentuated at low extracellular K+
Hypokalemia Decreases IKr Prolongs APD](https://image.slidesharecdn.com/cap2recovered-190324101030/75/Cardiac-action-potential-30-2048.jpg)




![DAD
Increased cytosolic Ca++
Digitalis toxicity diastolic
Ca++ release from SR
β-adrenoceptor stimulation
Low extracellular [K+]
Ischemia
Precipitated in background of
rapid HR](https://image.slidesharecdn.com/cap2recovered-190324101030/75/Cardiac-action-potential-37-2048.jpg)




![TEATMENT
Life style modification
b blockers in LQTS clinical diagnosis (ecg) [ may be
given in pts with molecular diagnosis alone]
Sodium channel blockers (mexiletine, flecainide or
ranolazine) add-on therapy LQTS3 patients with a
QTc > 500 ms.
PPI in cases with sustained pause dependent VT +/-
QT prolongation
ICD in survivors of cardiac arrest, may be given in b
blocker resistant, considered in high risk groups
[LQT2, LQT3, QT>500ms]
Left cardiac sympathetic denervation considered for
symptomatic b blocker resistant]](https://image.slidesharecdn.com/cap2recovered-190324101030/75/Cardiac-action-potential-42-2048.jpg)



![SQTS
Remarkably accelerated repolarization that is
reflected in a shorter-than-normal QTc [<320
msec]
Susceptibility to arrhythmias and sudden death
[paroxysmal atrial fibrillation, syncope, and an
increased risk for SCD]
Syncope 25% pts, Family history of SCD 30% pts,
AF in 1/3rd.
Most often during Rest or Sleep.
5 genes
Gain of function mutations in K channel- KCNH2 [IKr]
(SQT1), KCNQ1 [IKs] (SQT2), and KCNJ2 [IK1] (SQT3)
Loss of function mutations in ICaL -CACNA1C (SQT4)
and CACNB2b (SQT5)](https://image.slidesharecdn.com/cap2recovered-190324101030/75/Cardiac-action-potential-46-2048.jpg)