CHEMICAL BONDS AND WATER
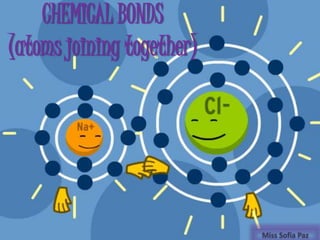
- 1. CHEMICAL BONDS (atoms joining together) Miss Sofia Paz
- 2. CHEMICAL BONDS Process of SHARING or TRANSFERRING electrons (attraction) Ionic bond Covalent Bond
- 3. CHEMICAL BONDS Electronegativity values of two atom are different... Ionic bonds are formed, electrons are transferred Electronegativity values of two atom are similar.. COVALENT 1.Nonpolar Covalent bonds form when the electronegativity values are very similar. 2.Polar Covalent bonds form when the electronegativity values are a little further apart. Covalent bonds form between two non-metal atoms. IONIC BOND COVALENT BOND
- 4. Ionic bond One atom transfers an electron to another atom (Electronegativity values of two atom are different)
- 6. Atoms colide and Cl strips Na’s outer electron Cl has 8 electrons on its outer level Na has 8 on its outer level Electric balances have changed.
- 7. Two atoms share electrons (Electronegativity values of two atom are similar) Covalent Bonds
- 10. Covalent Bonds
- 11. WATER Polar Covalent Bond Oxygen end of the molecule has a slight negative charge The end with the two hydrogen atoms is slightly positive.
- 12. Two or more atoms held together by covalent bonds. # and types of atoms in a molecule # and types of atoms in a molecule How atoms are linked by bonds MOLECULES Atoms and complexes connected by non-covalent bonds such as hydrogen bonds or ionic bonds are generally not considered single molecules
- 13. Chemical reactions Rearrangement of molecules. Existing bonds break and new ones form, resulting in the formation of new substances. Release more energy than they absorb Absorb more energy than they release. EXOTHERMIC REACTION Endothermic reaction
- 14. Chemical reactions do not create or destroy atoms, but only rearrange them. These rearrangements usually involve breaking chemical bonds in reactants and forming new bonds in products. Exothermic reaction Endothermic reaction sunlight + 6CO2(g) + H2O(l) = C6H12O6(aq) + 6O2(g) Release more energy than they absorb Absorb more energy than they release Chemical reactions
- 15. The structure of Water The end with the two hydrogen atoms is slightly positive. Oxygen end of the molecule has a slight negative charge POLAR MOLECULE!!
- 16. The electromagnetic attractive interaction between polar molecules, in which hydrogen (H) is bound to a highly electronegative atom, such as nitrogen (N), oxygen (O) or fluorine (F). Hydrogen bond is not a true bond but a strong dipole- dipole attraction, and should not be confused with a covalent bond
- 17. Q:How can water molecules be compared to magnets? A:Both water molecules and magnets have opposite poles that cause them to be attracted to each other in a specific orientation.
- 18. Life-Supporting Properties of WATER WATER H2O Cohesion & Adhesion Temperature Moderation Dissolve Substances Low density of ice
- 19. Life-Supporting Properties of WATER WATER H2O Cohesion & Adhesion Temperature Moderation Dissolve Substances Low density of ice
- 21. Life-Supporting Properties of WATER WATER H2O Cohesion & Adhesion
- 22. Life-Supporting Properties of WATER WATER H2O Temperature Moderation
- 23. Life-Supporting Properties of WATER WATER H2O Dissolve Substances
- 24. Life-Supporting Properties of WATER WATER H2O Low density of ice
