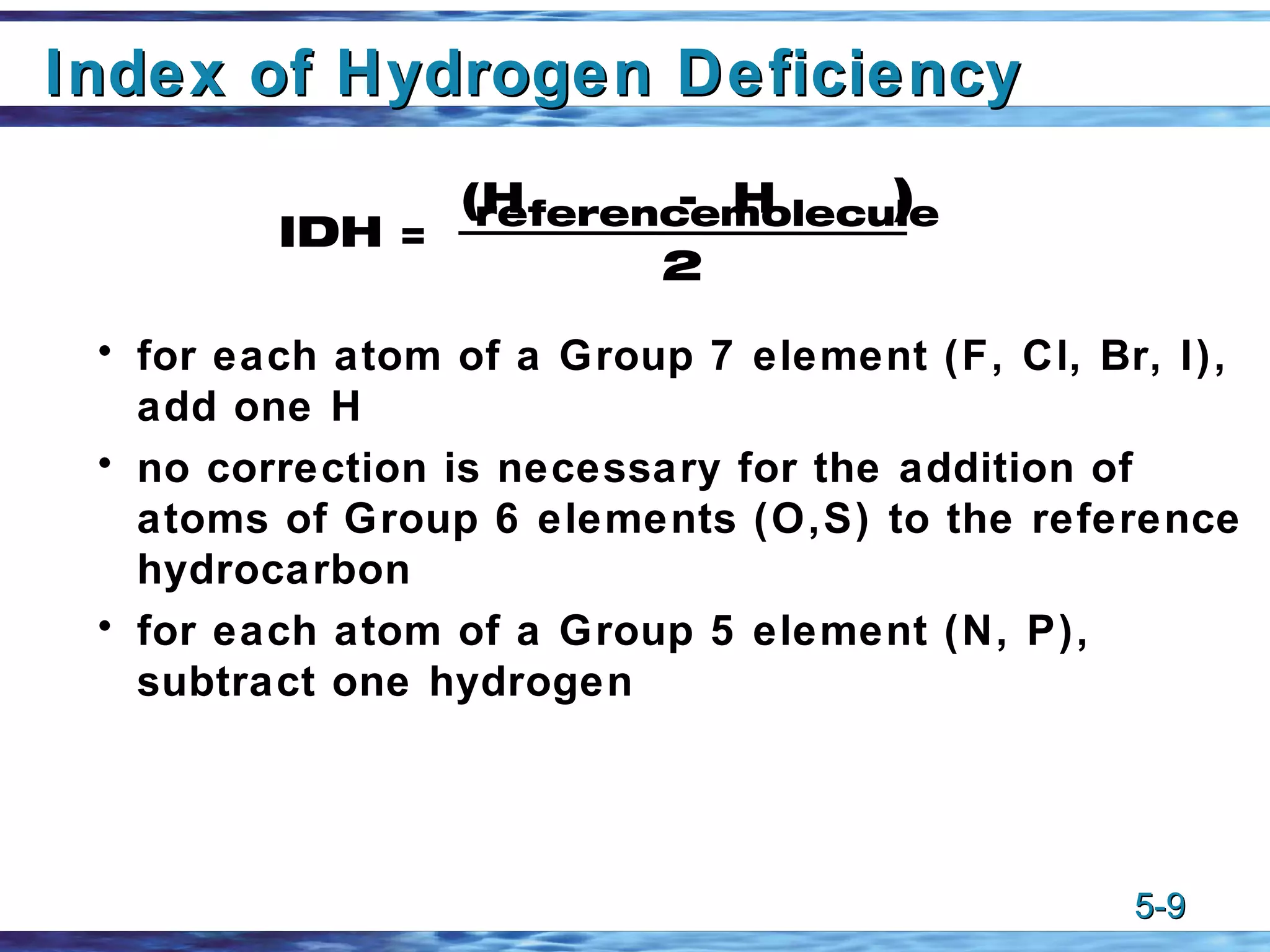
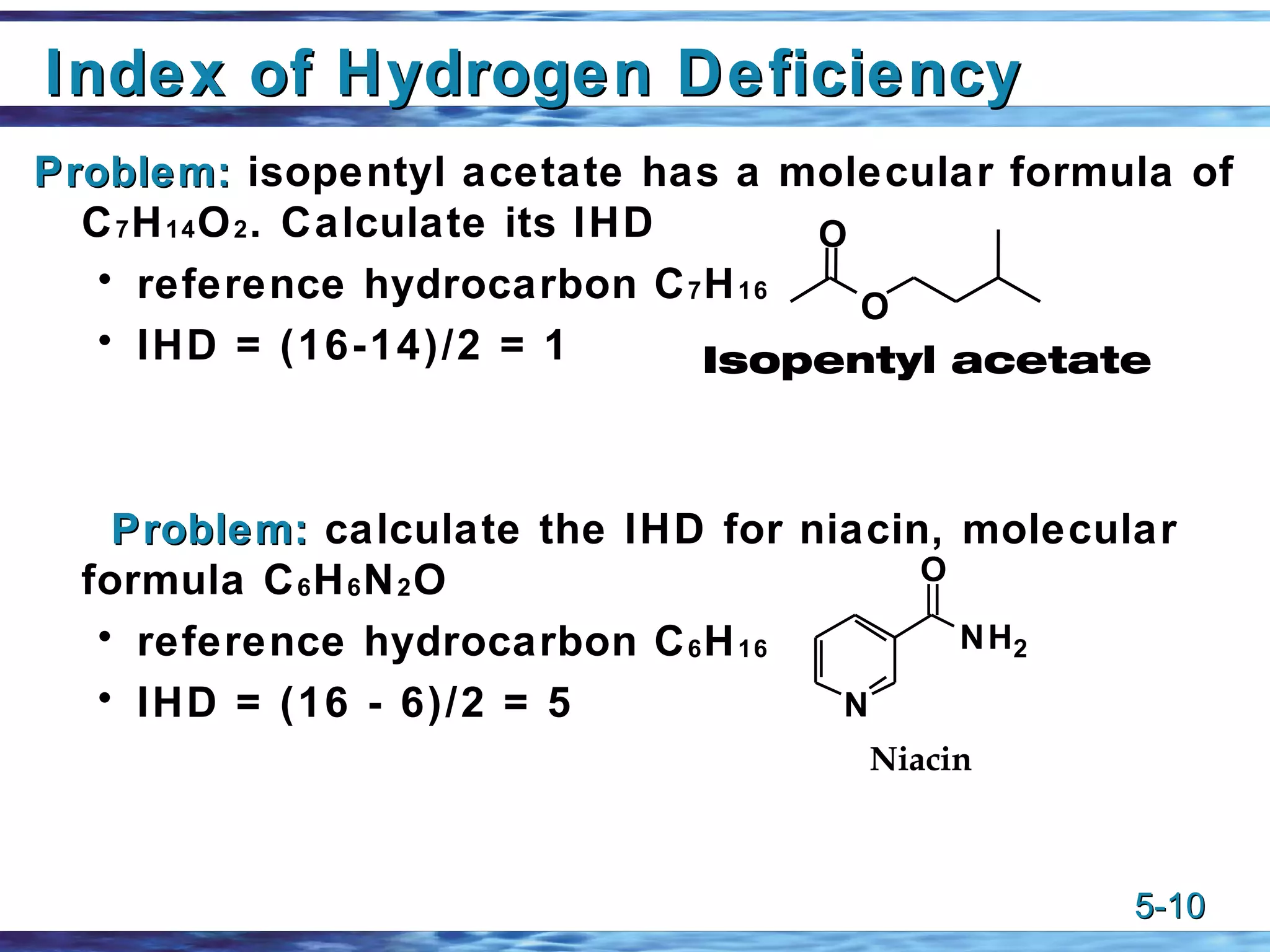
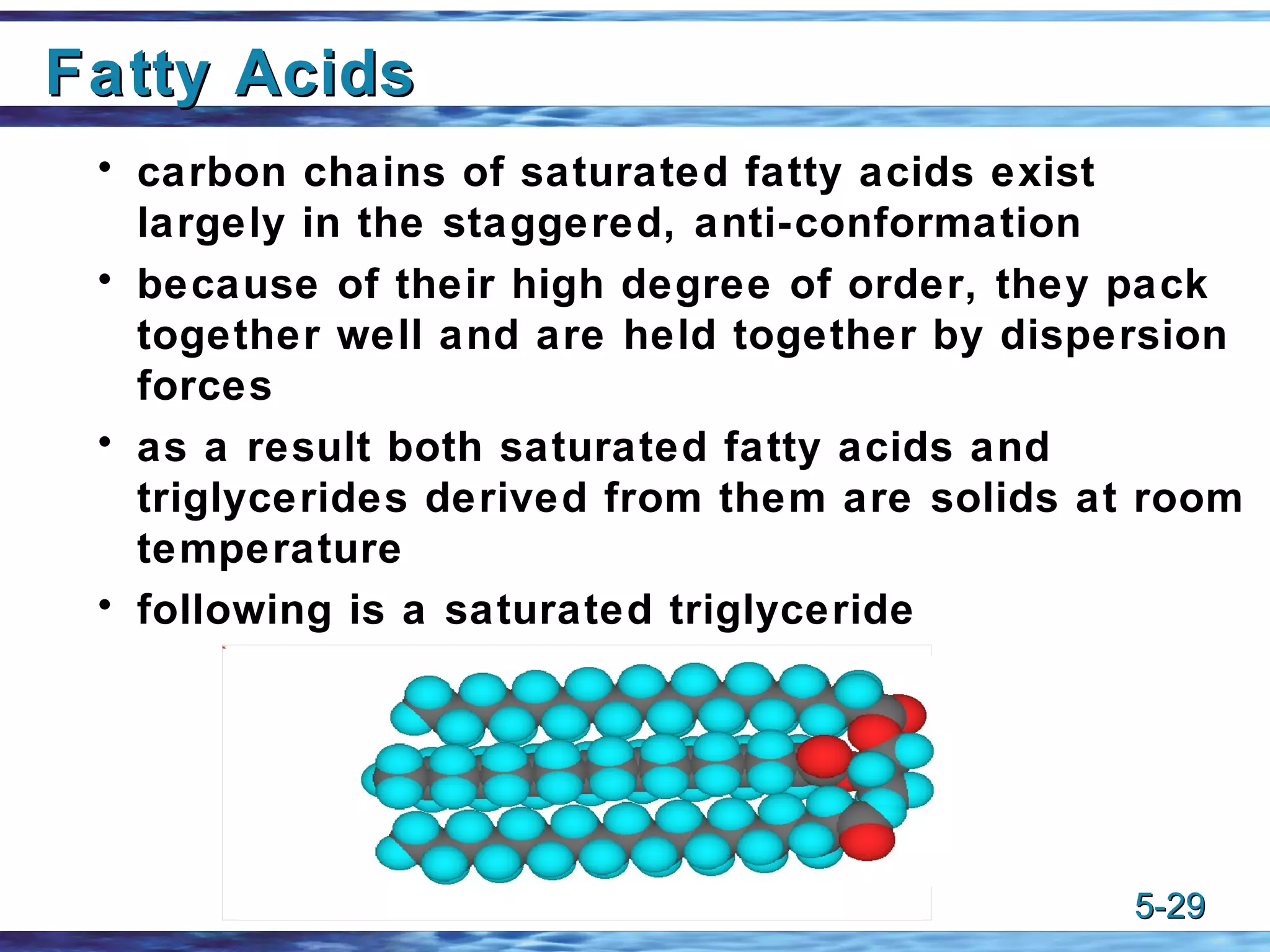
1) Alkenes are unsaturated hydrocarbons that contain one or more carbon-carbon double bonds. The general formula for alkenes is CnH2n.
2) A carbon-carbon double bond consists of one sigma bond and one pi bond formed from the overlap of sp2 hybrid orbitals and unhybridized p orbitals.
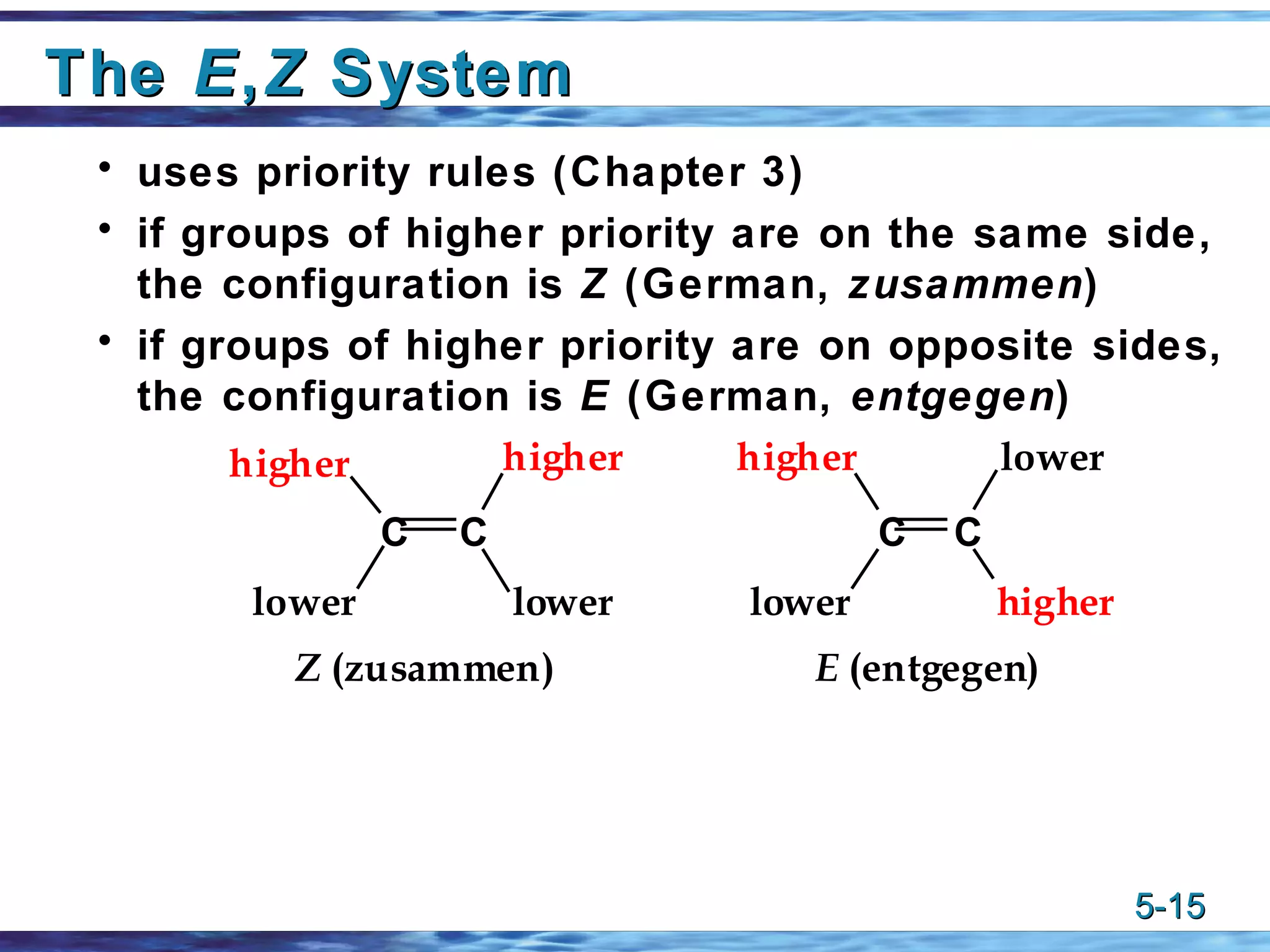
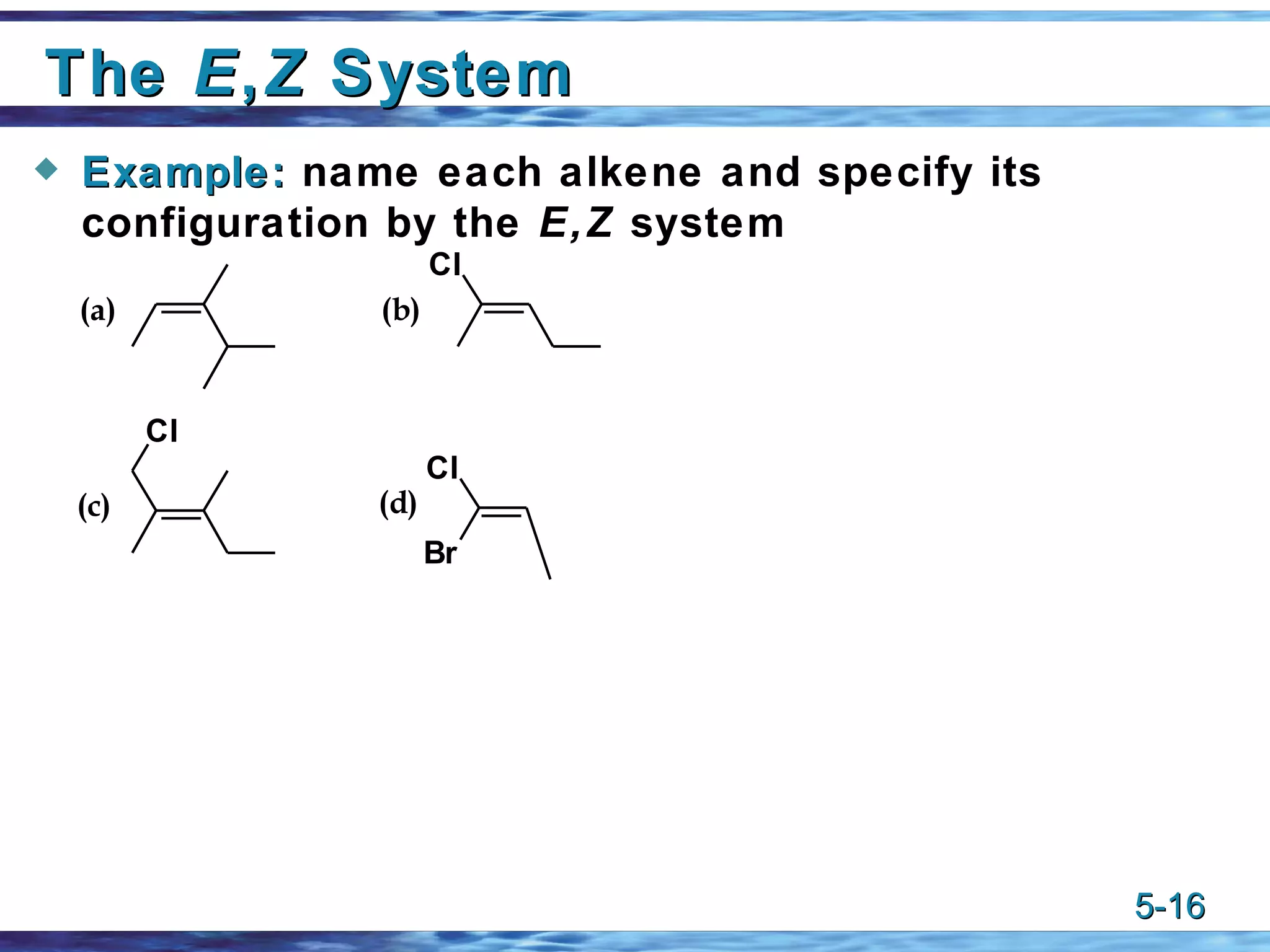
3) The cis-trans or E-Z systems are used to describe the stereochemistry of alkenes, including cycloalkenes and dienes, based on the orientation of substituents around the double bond(s).