Embed presentation
Download as PDF, PPTX











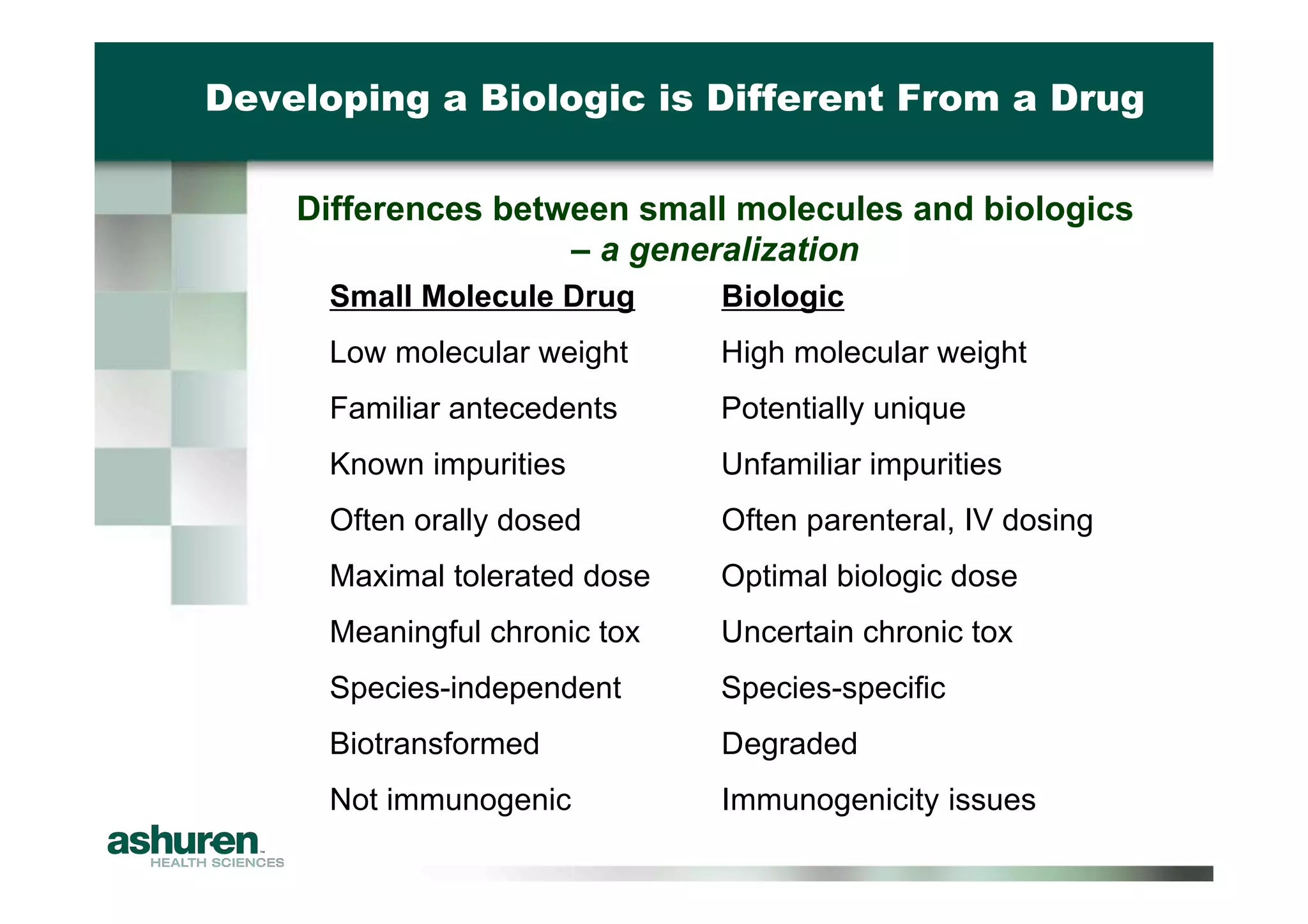


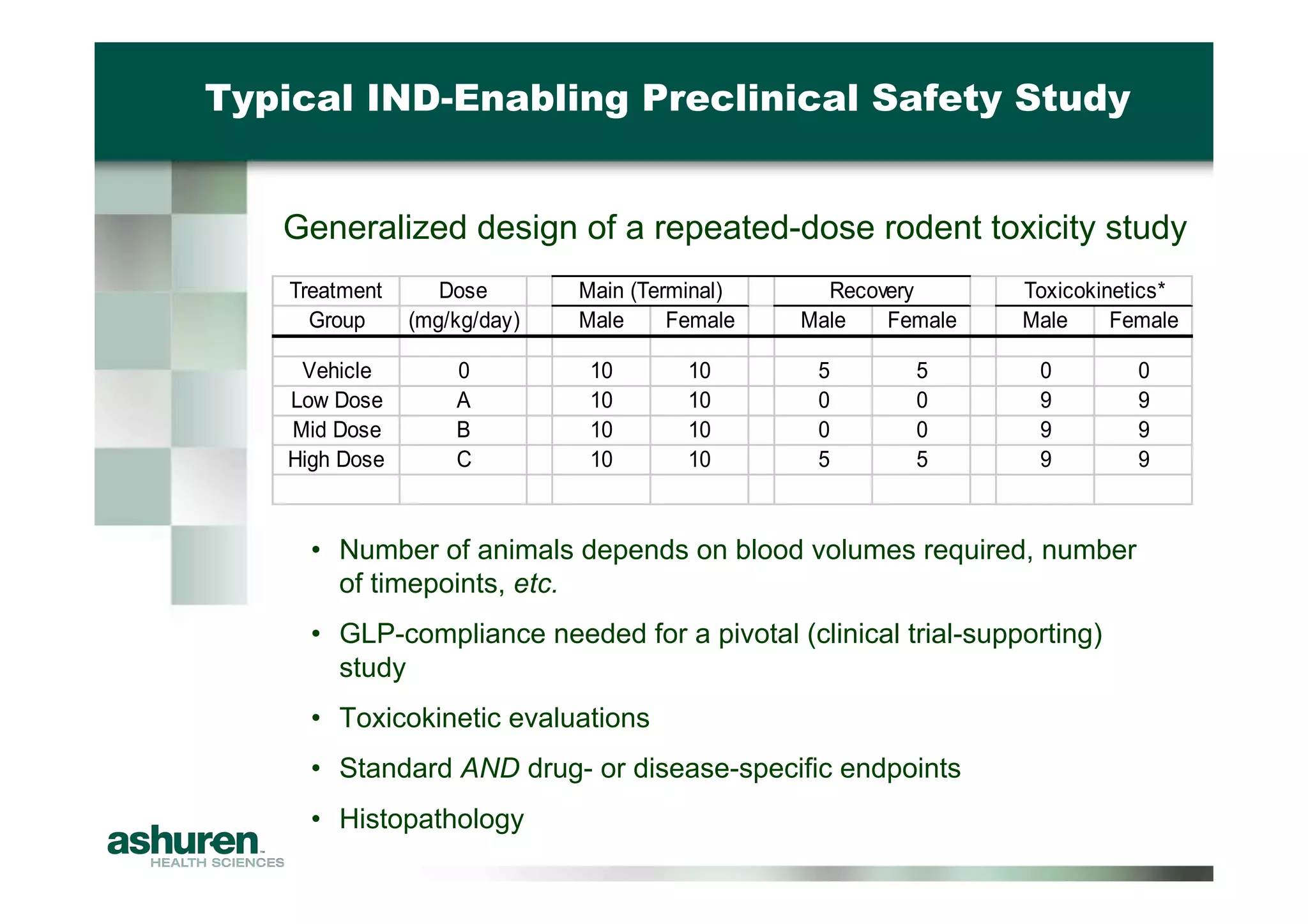

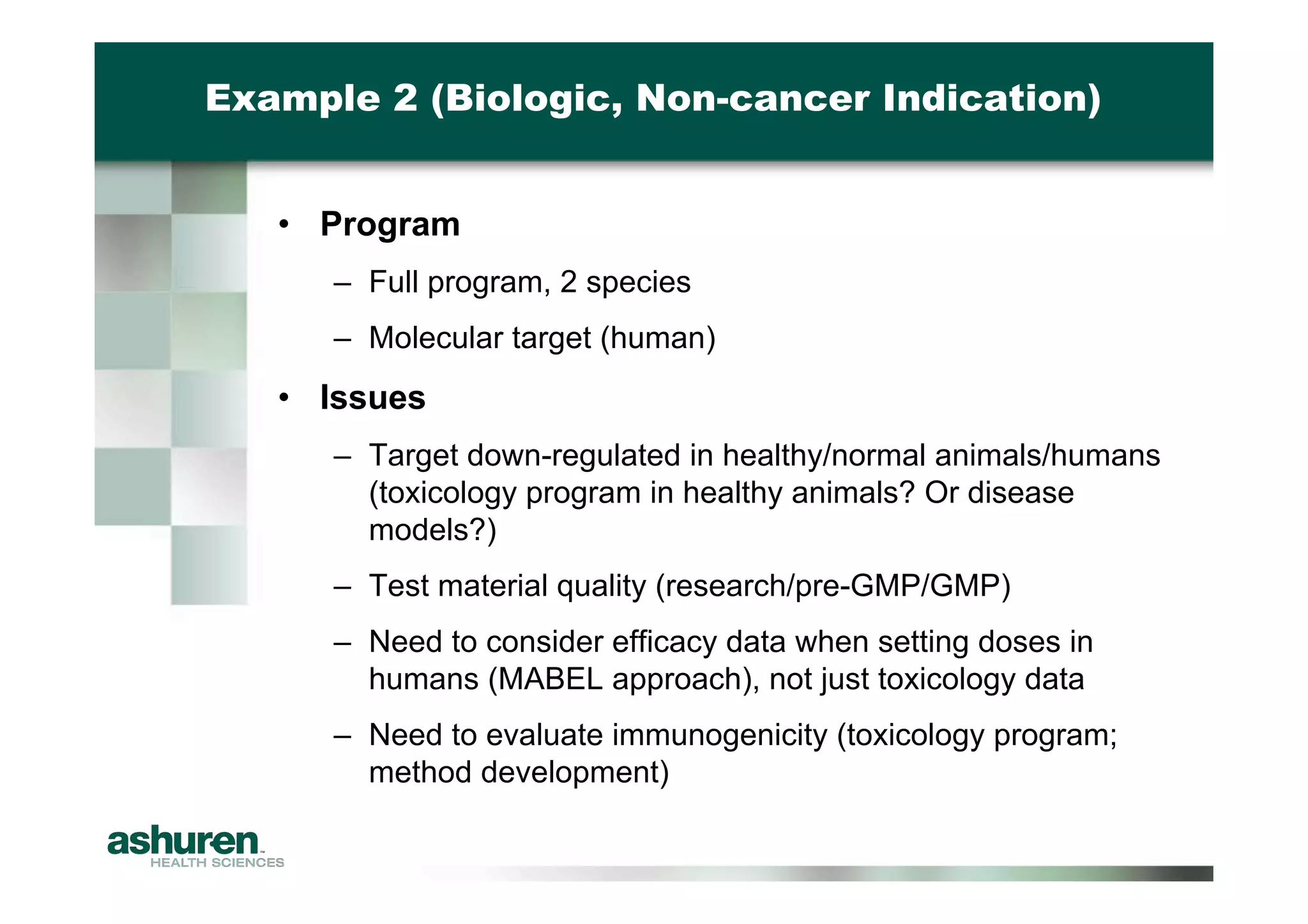






This document provides guidance for emerging biotech companies on planning and conducting preclinical development programs to support a First-in-Human clinical trial. It outlines typical timelines, challenges companies may face, requirements for an Investigational New Drug Application, and considerations for general toxicology programs and studies. Specific examples of preclinical development programs are also provided for a new cancer drug and a biologic for a non-cancer indication. Strategies are suggested to help companies have a successful preclinical program that results in a high-quality regulatory submission.
























