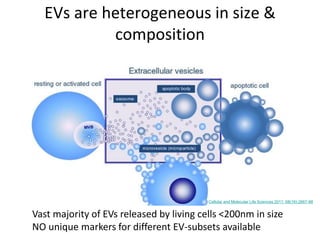
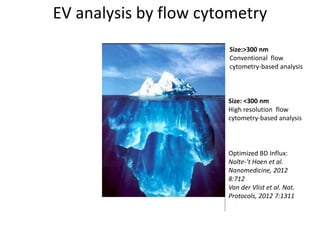
The document discusses the advancements and challenges in the analysis of extracellular vesicles (EVs) through flow cytometry, highlighting their potential as biomarkers and therapeutic agents. It addresses the technical difficulties in analyzing specific subsets of EVs in complex fluids, emphasizing the need for high throughput and high resolution techniques. Furthermore, it underscores the importance of standardization and comprehensive reporting in EV analysis to ensure reproducibility and comparison of results.