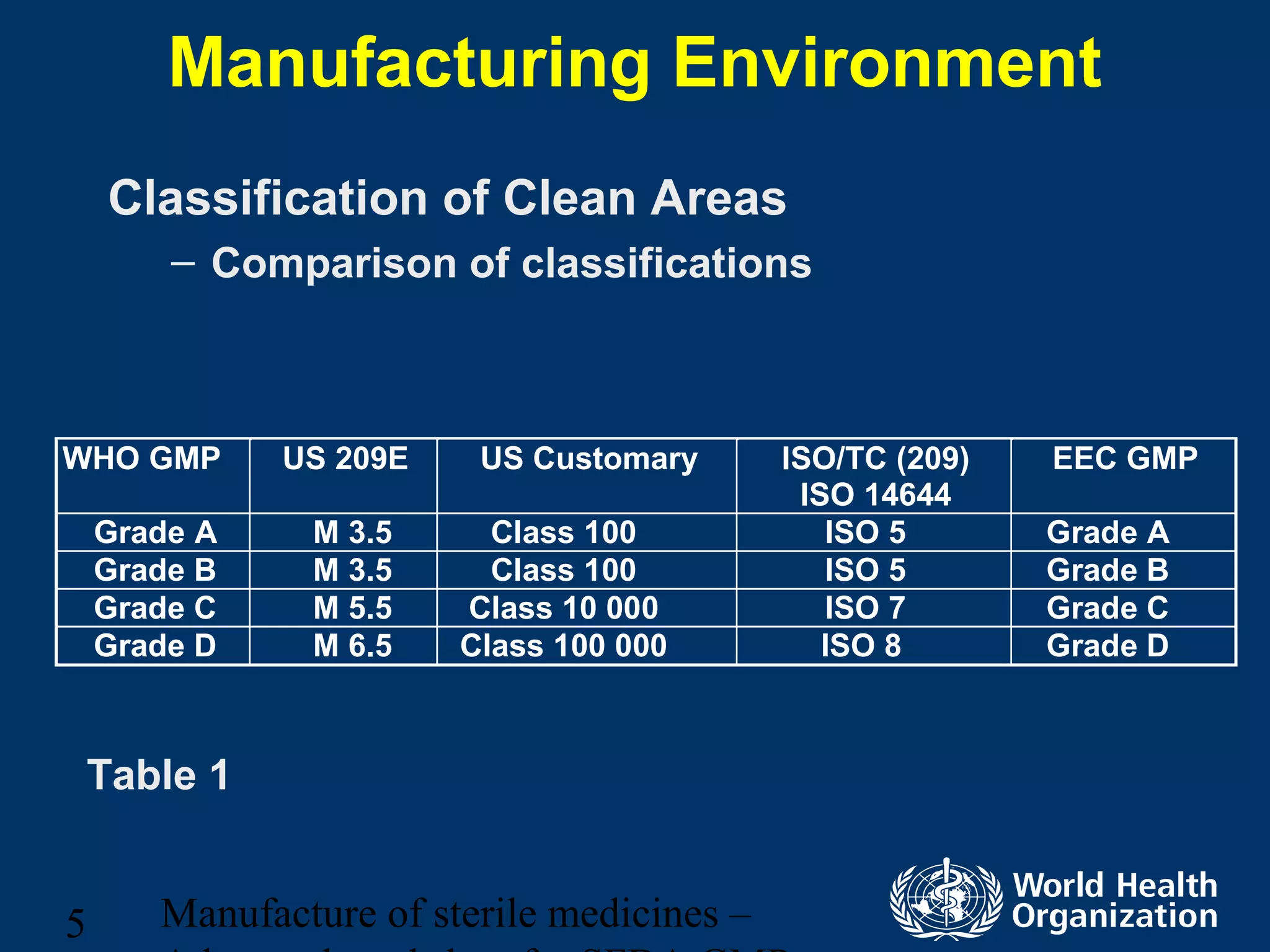
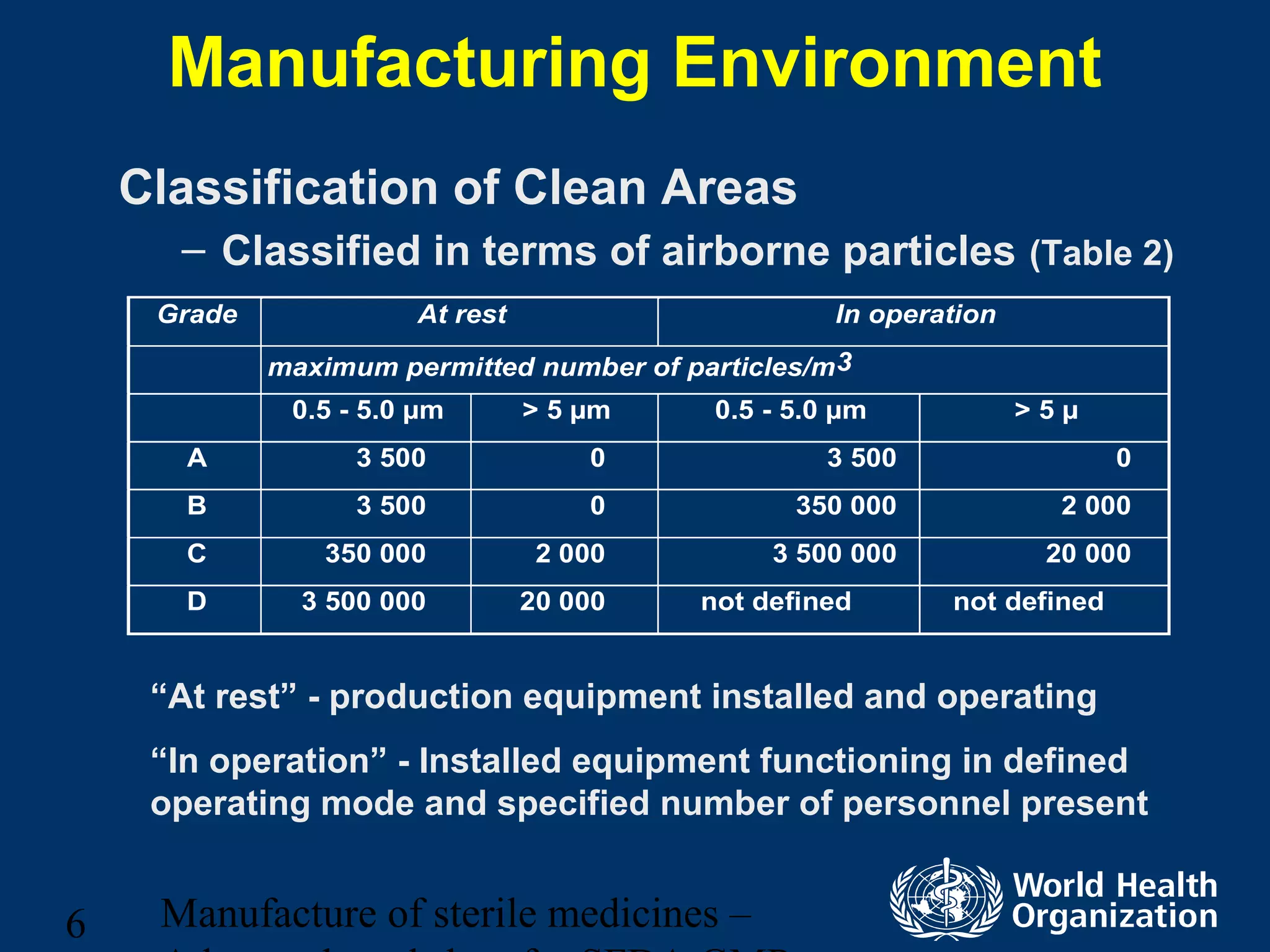
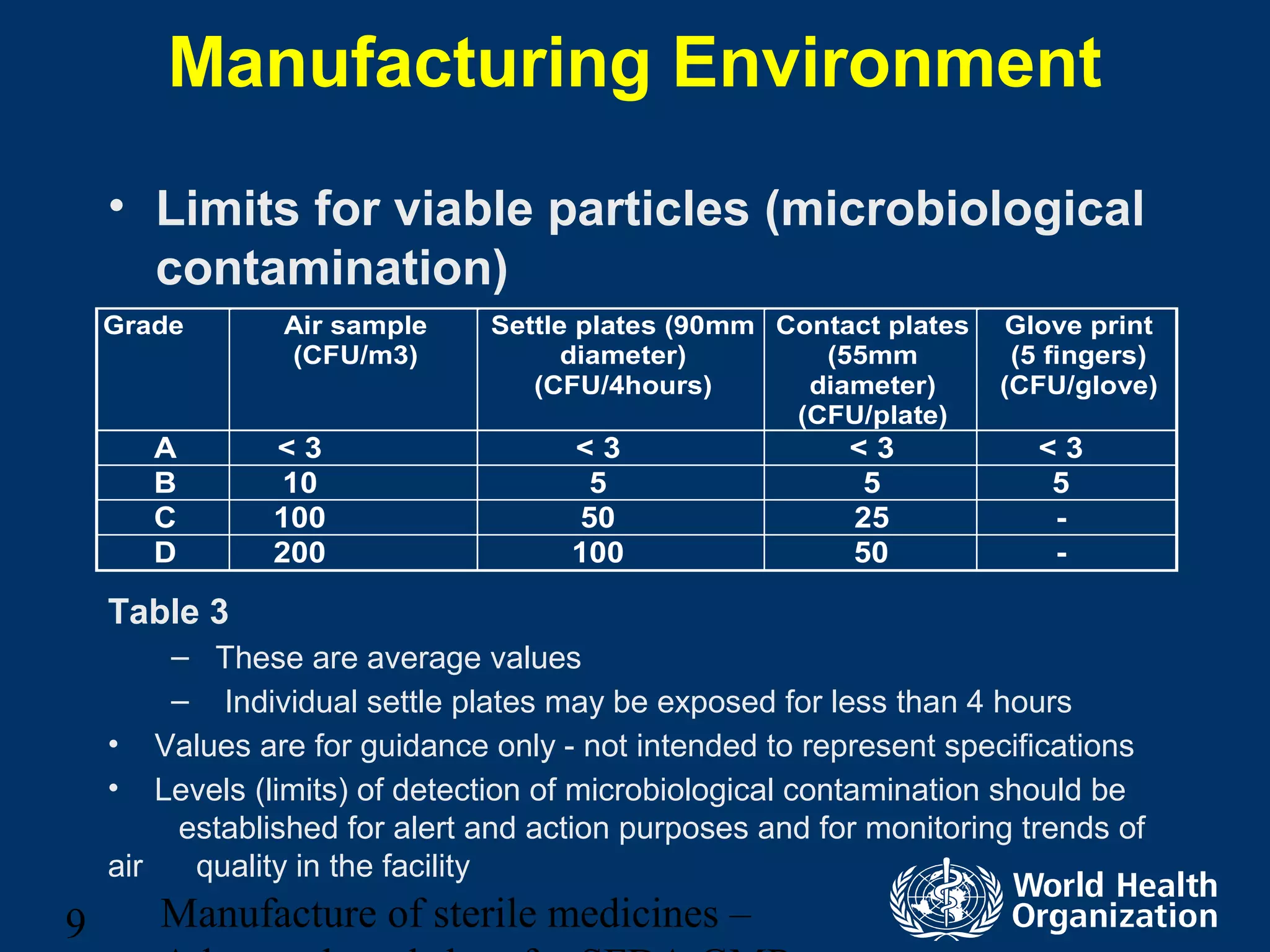
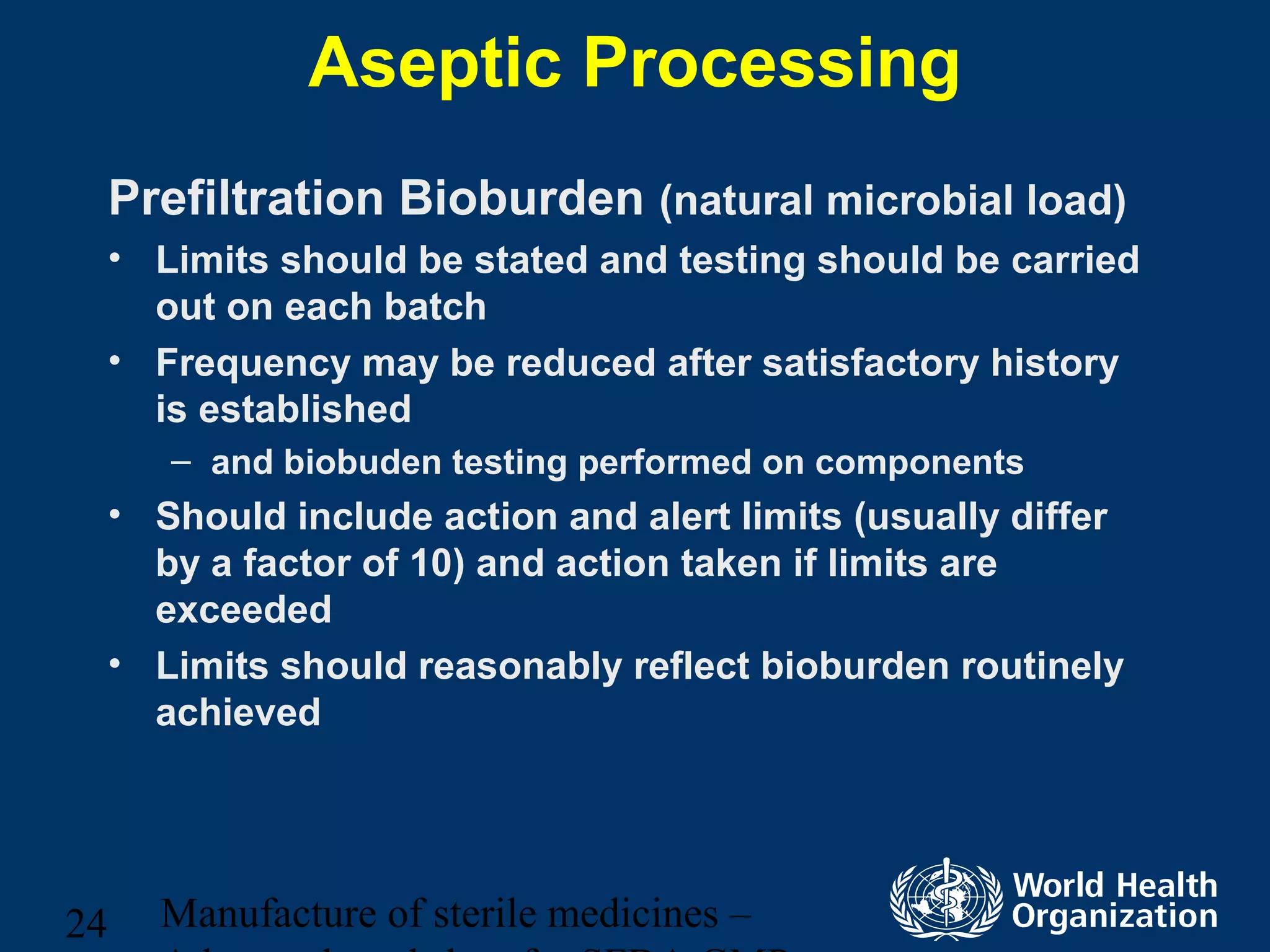
This document discusses aseptic processing for sterile pharmaceutical products. It cannot be terminally sterilized and must be aseptically prepared in a Grade A clean room. Key aspects covered include clean room classifications from Grades A to D, environmental monitoring of particulate matter and pressures, personnel training and hygiene, and the aseptic preparation and filtration of solutions to maintain sterility before filling into sterile containers.