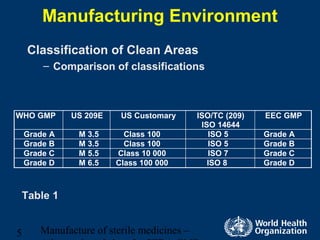
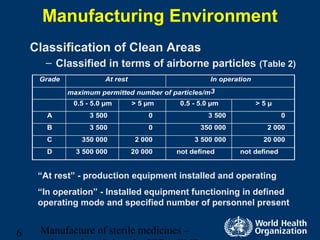
Certain pharmaceutical products such as injections must be sterile and cannot be terminally sterilized, requiring aseptic processing. Aseptic processing aims to maintain sterility by assembling sterile components in highly controlled clean areas. The document discusses manufacturing environments for aseptic processing, including classifications for clean areas, environmental monitoring, personnel requirements, and preparation of sterile solutions and equipment. It also covers validation of aseptic processes through media fills which simulate the entire manufacturing process.