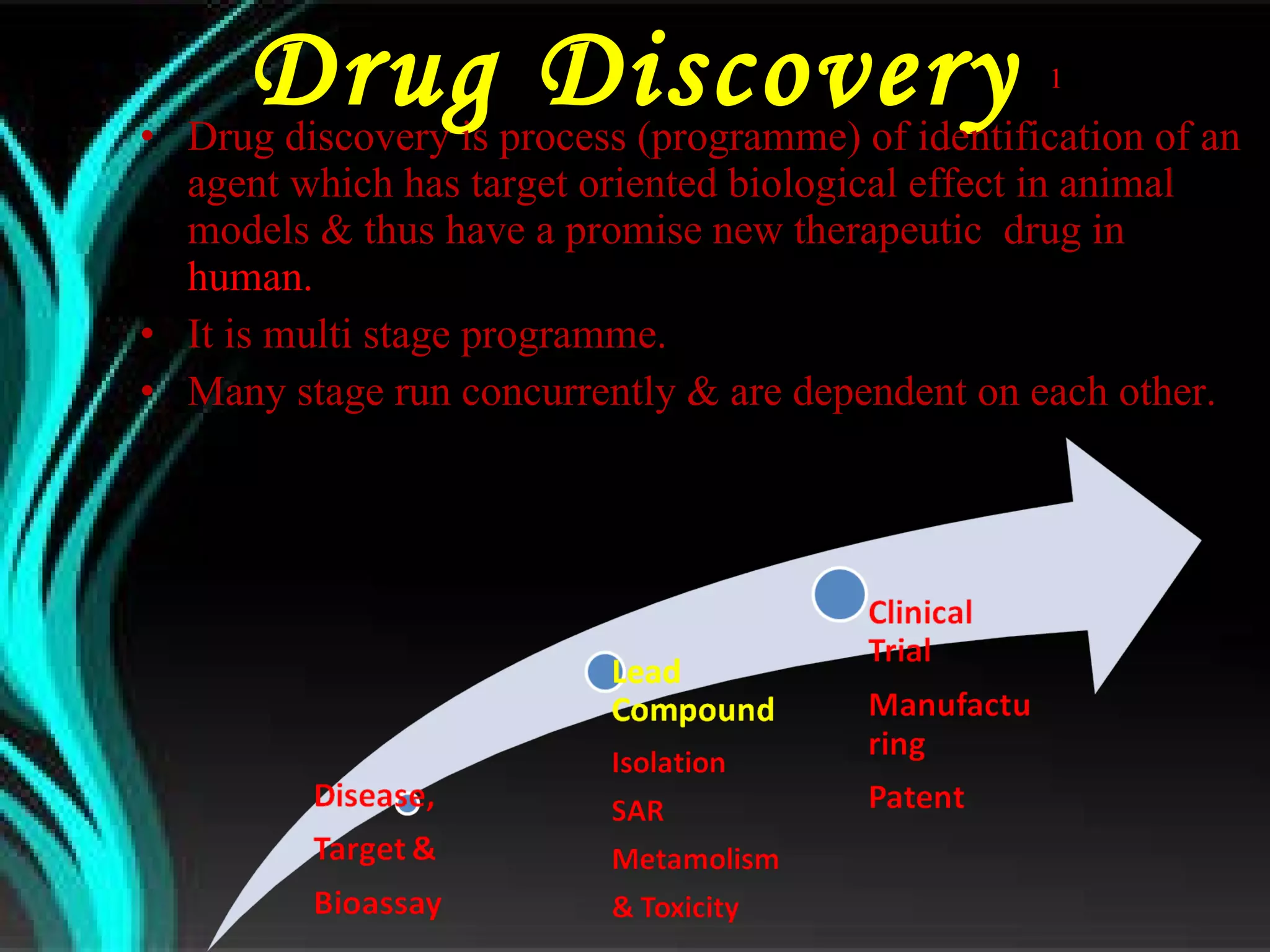
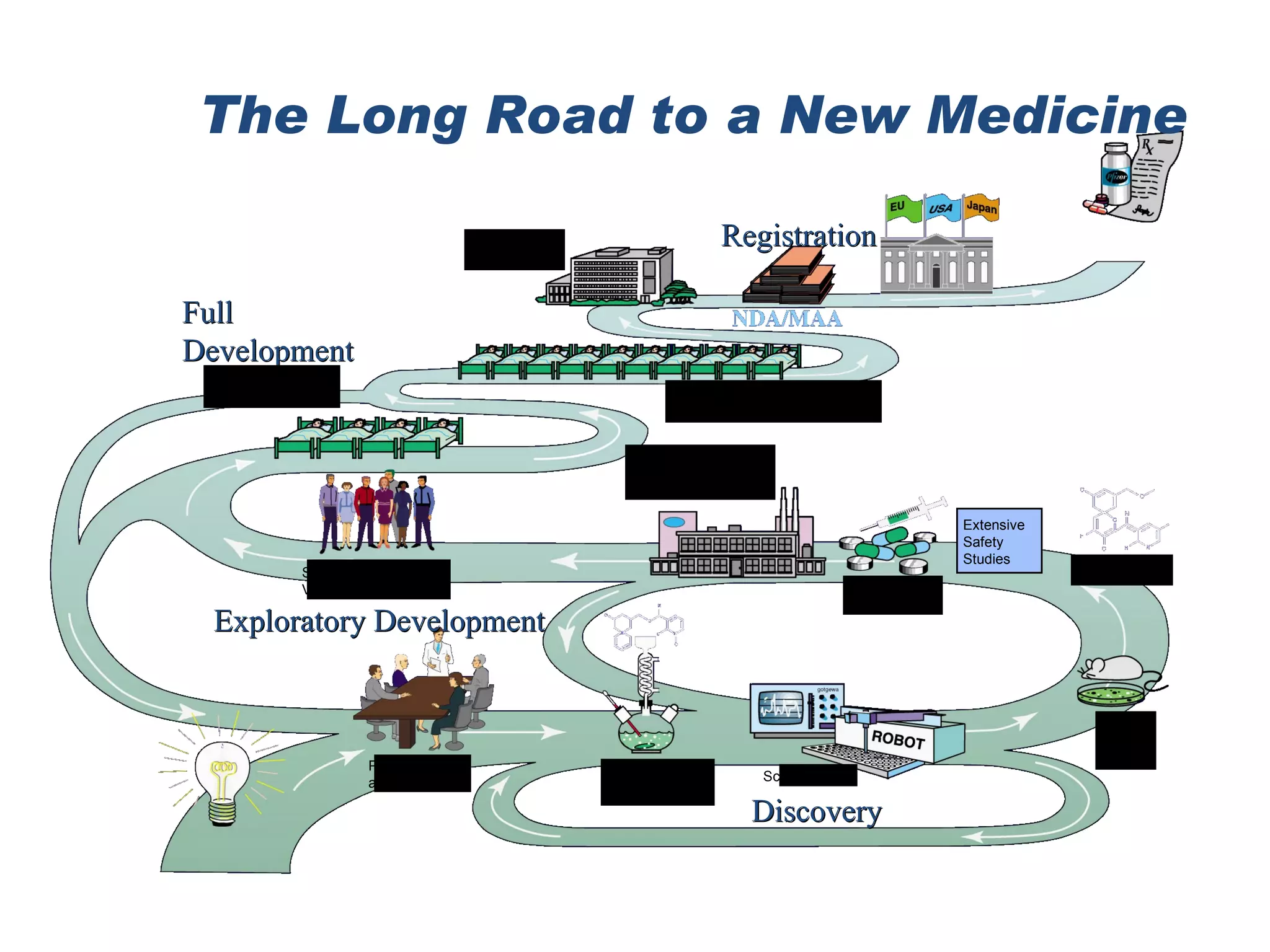
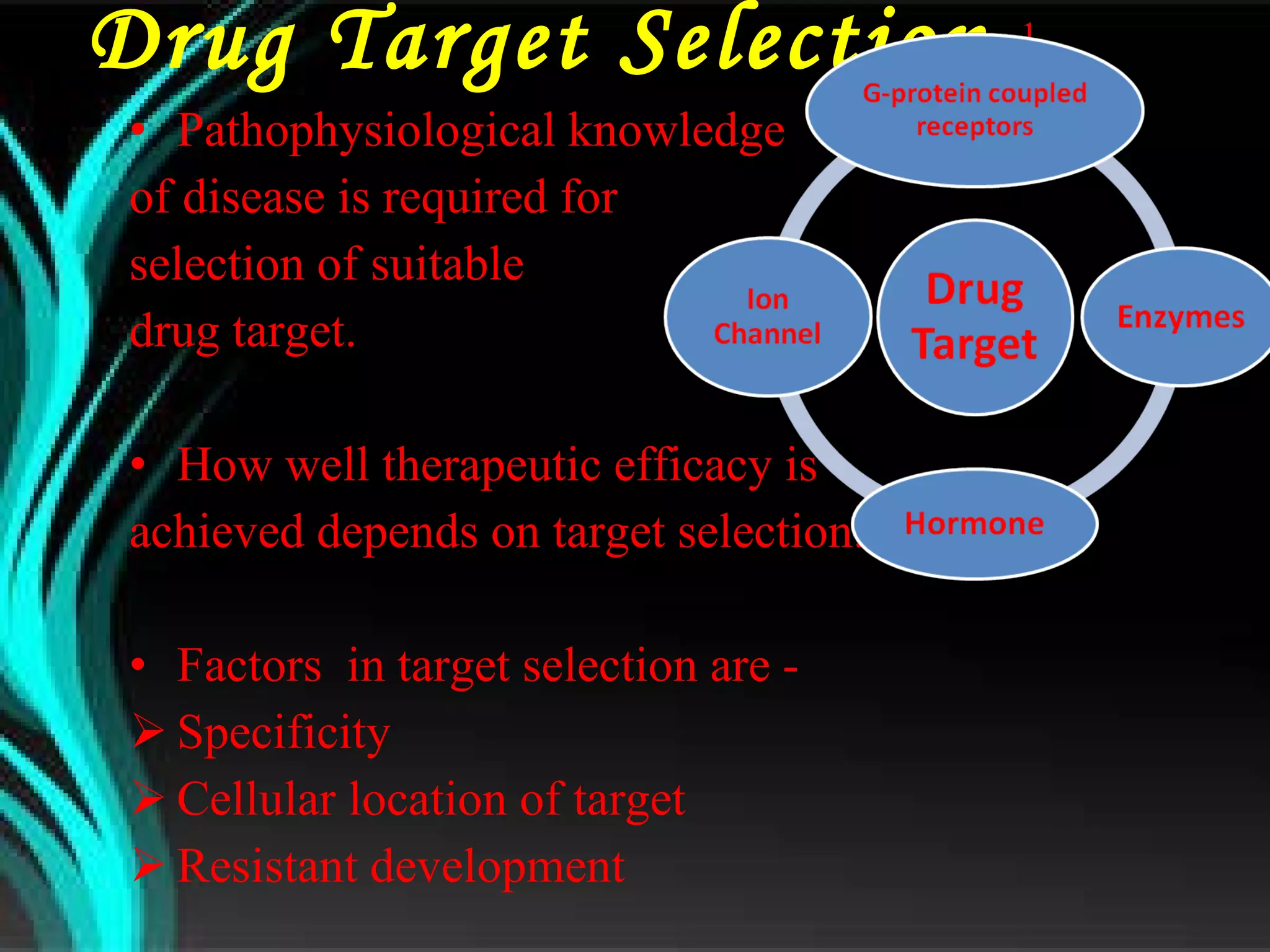
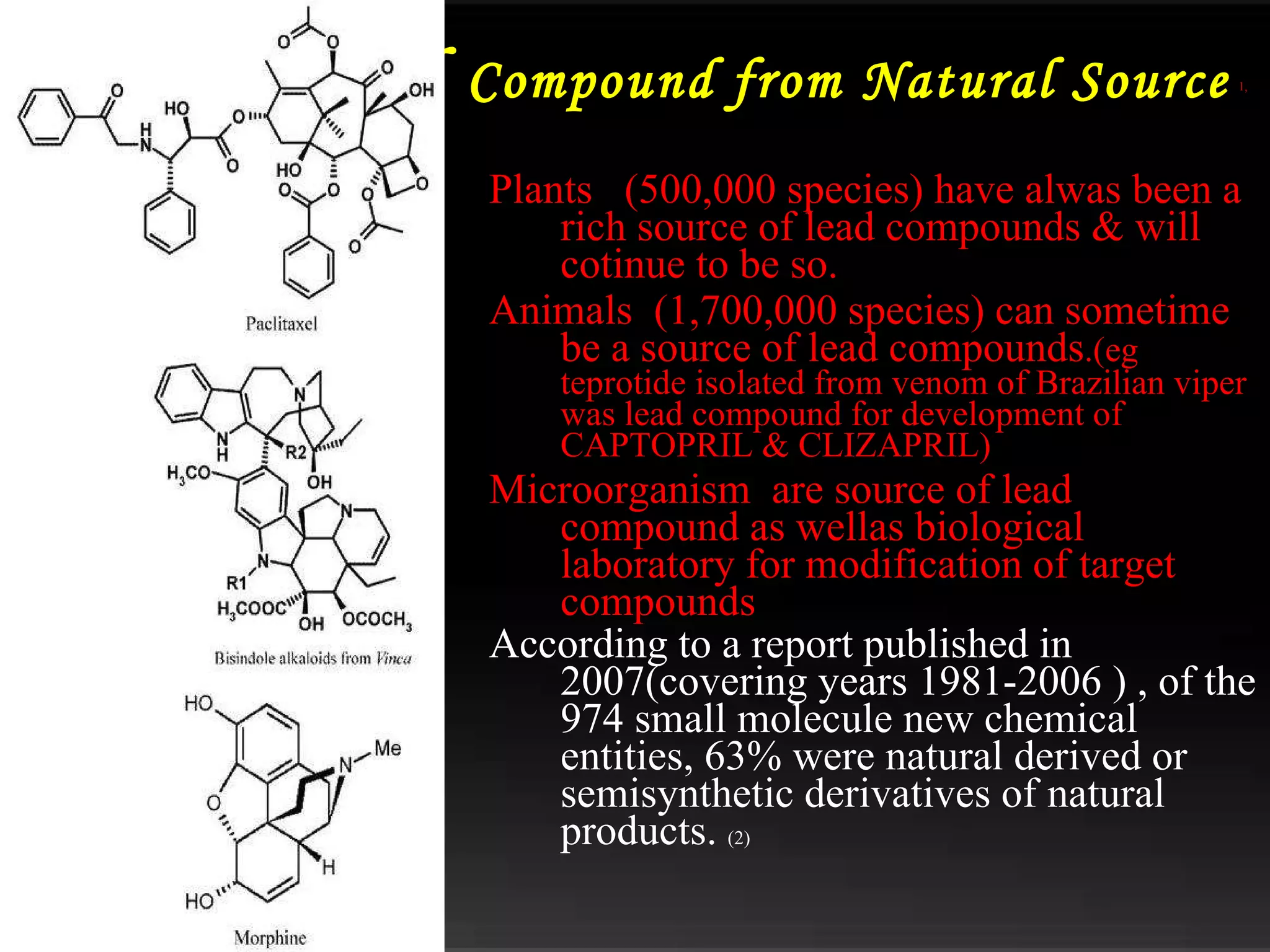
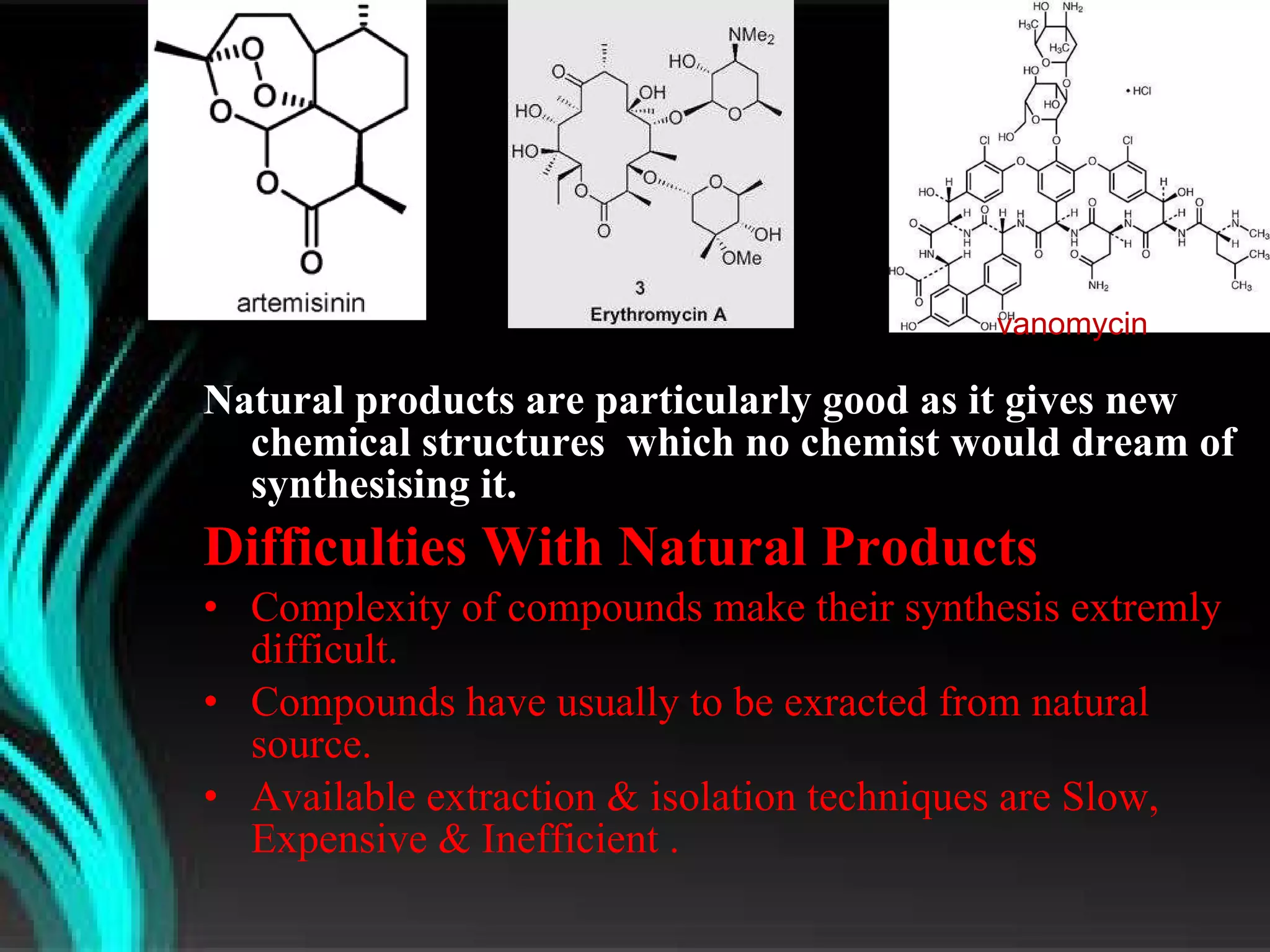
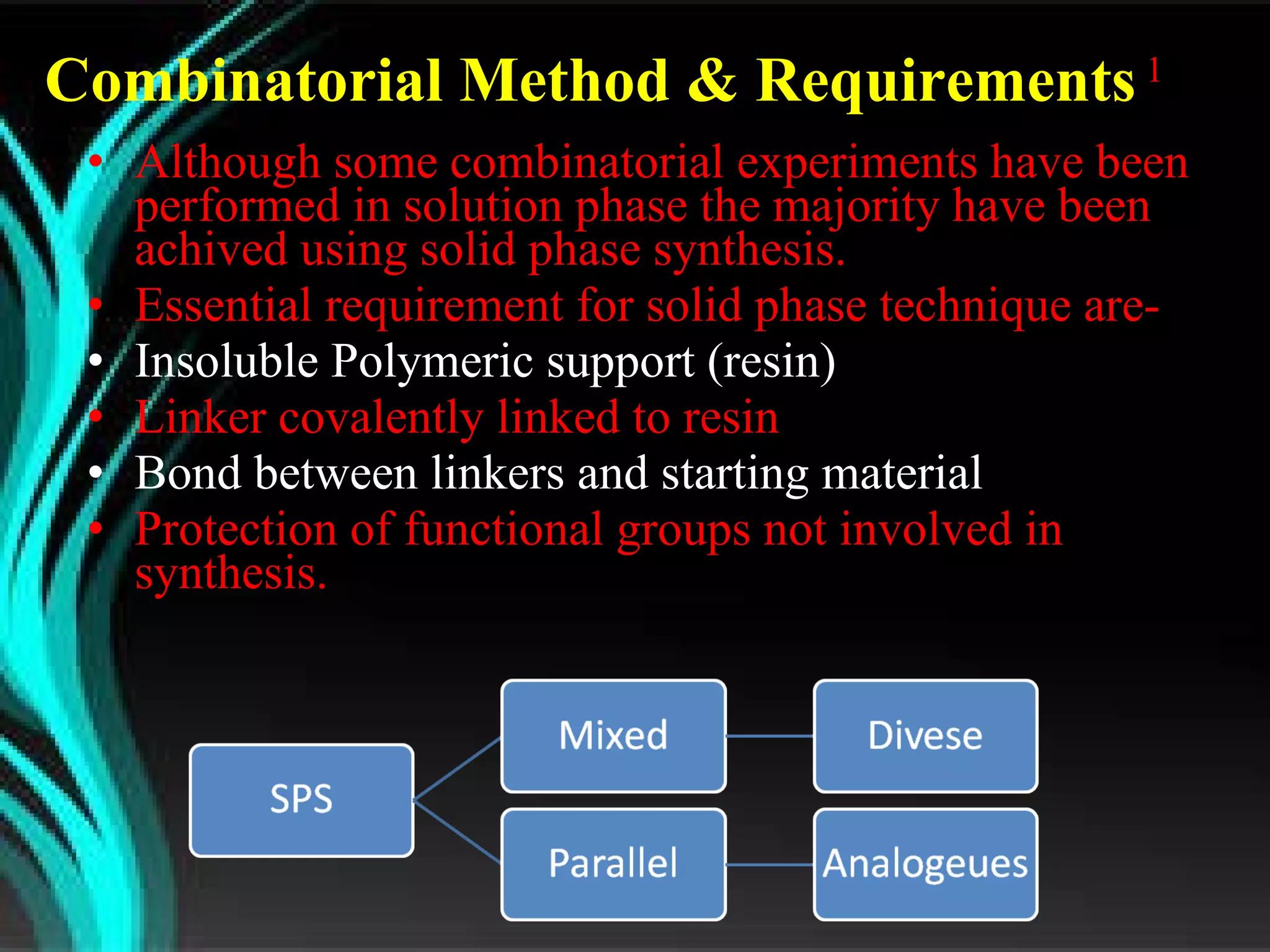
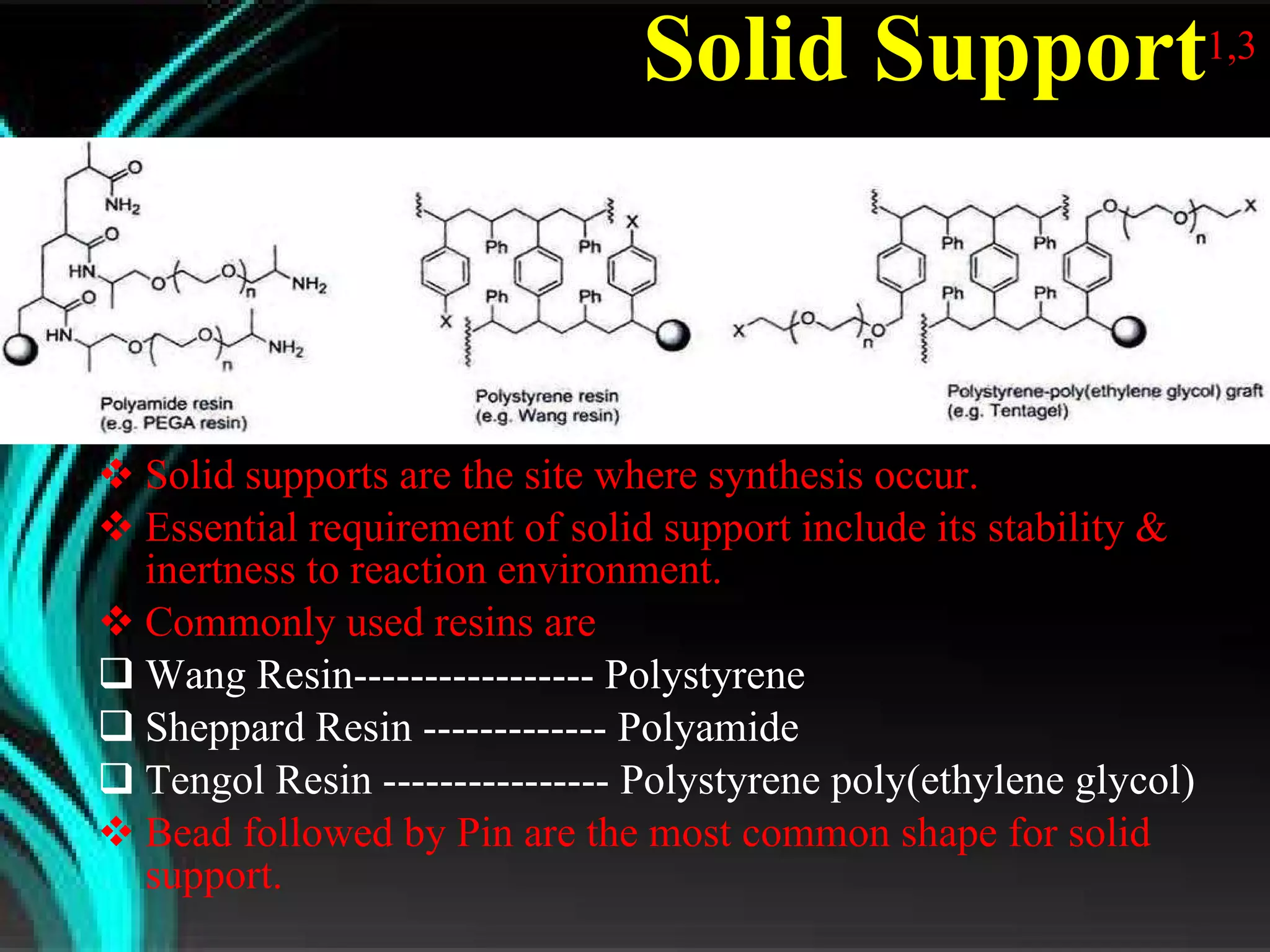
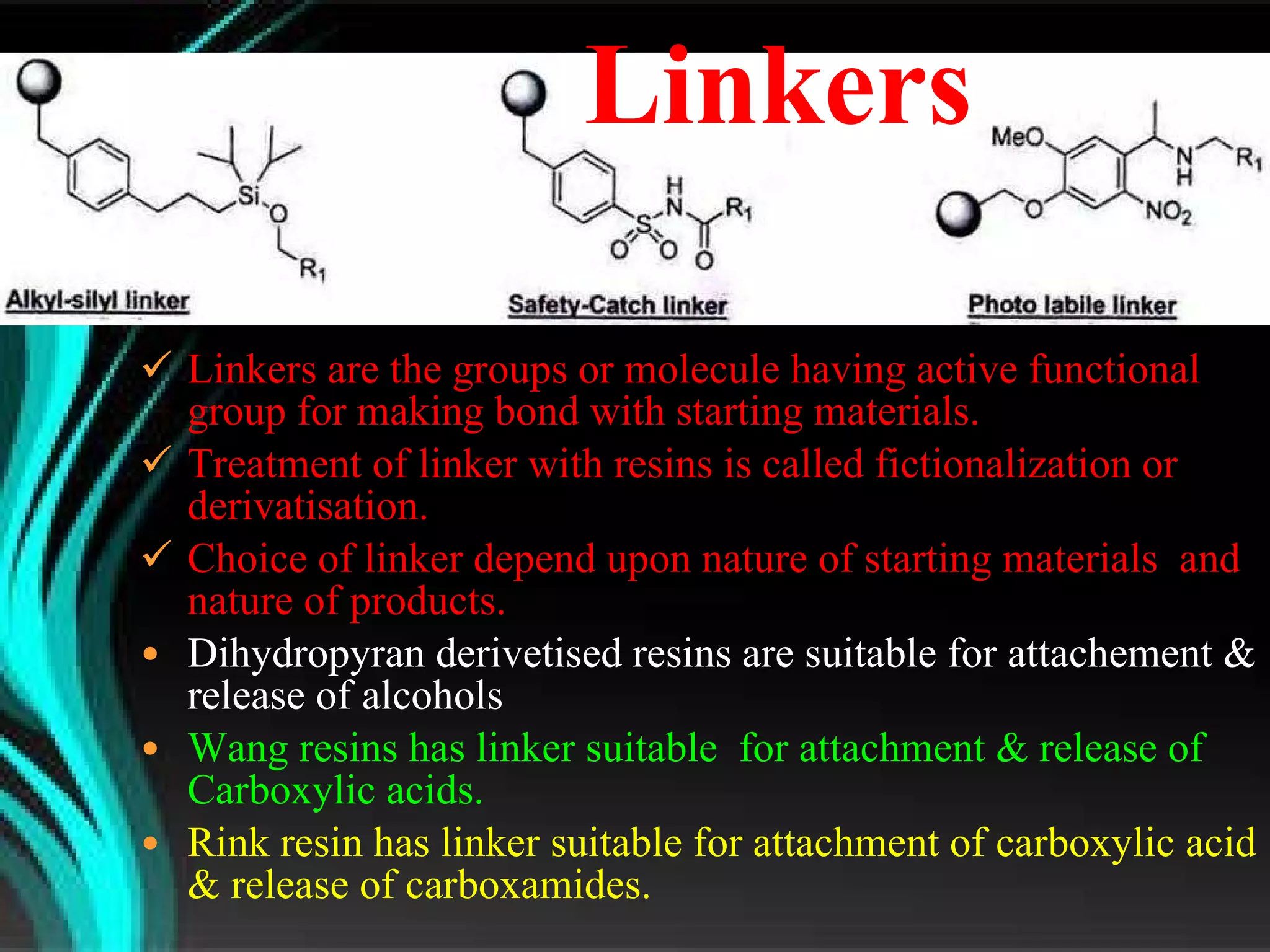
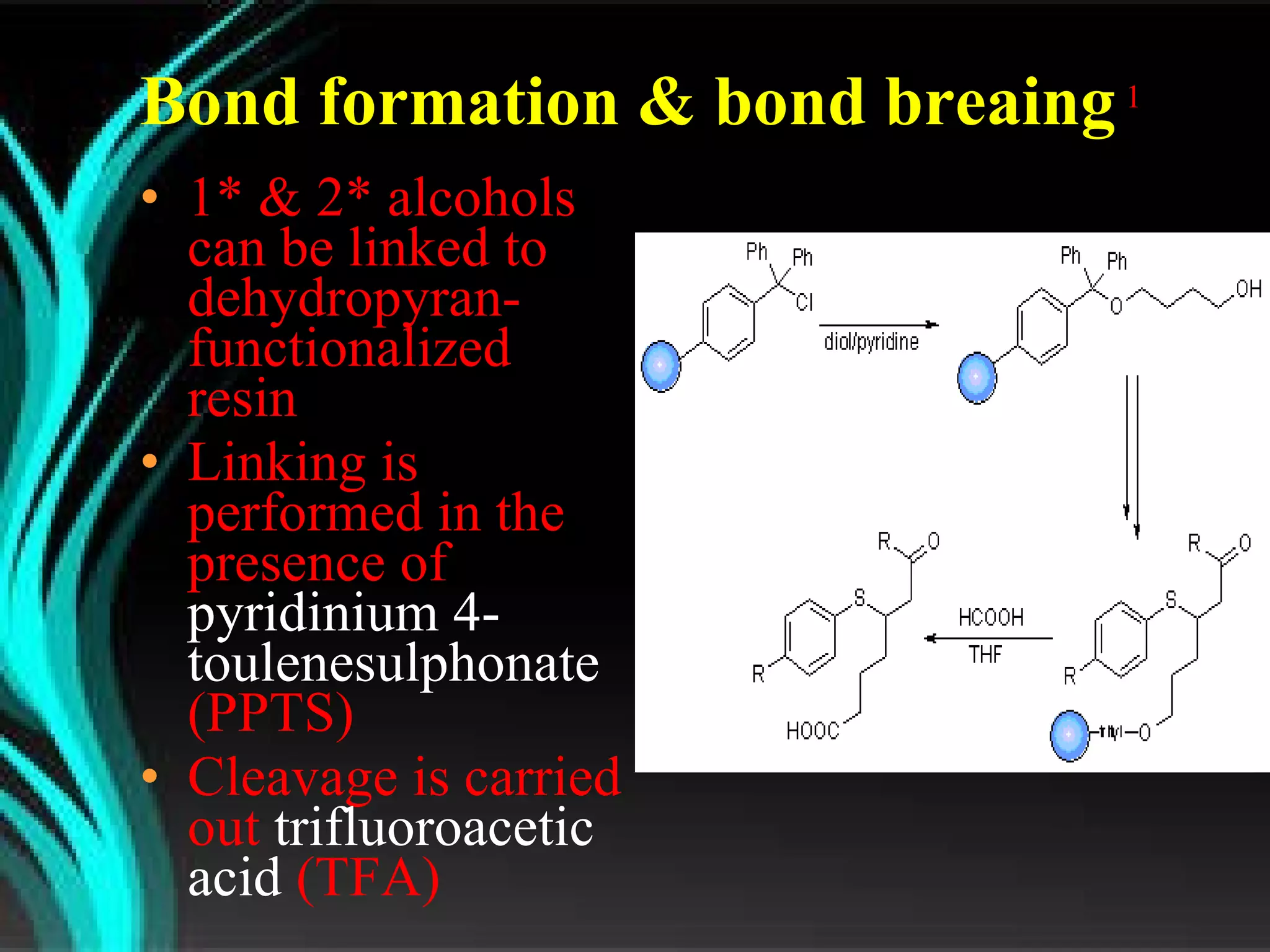
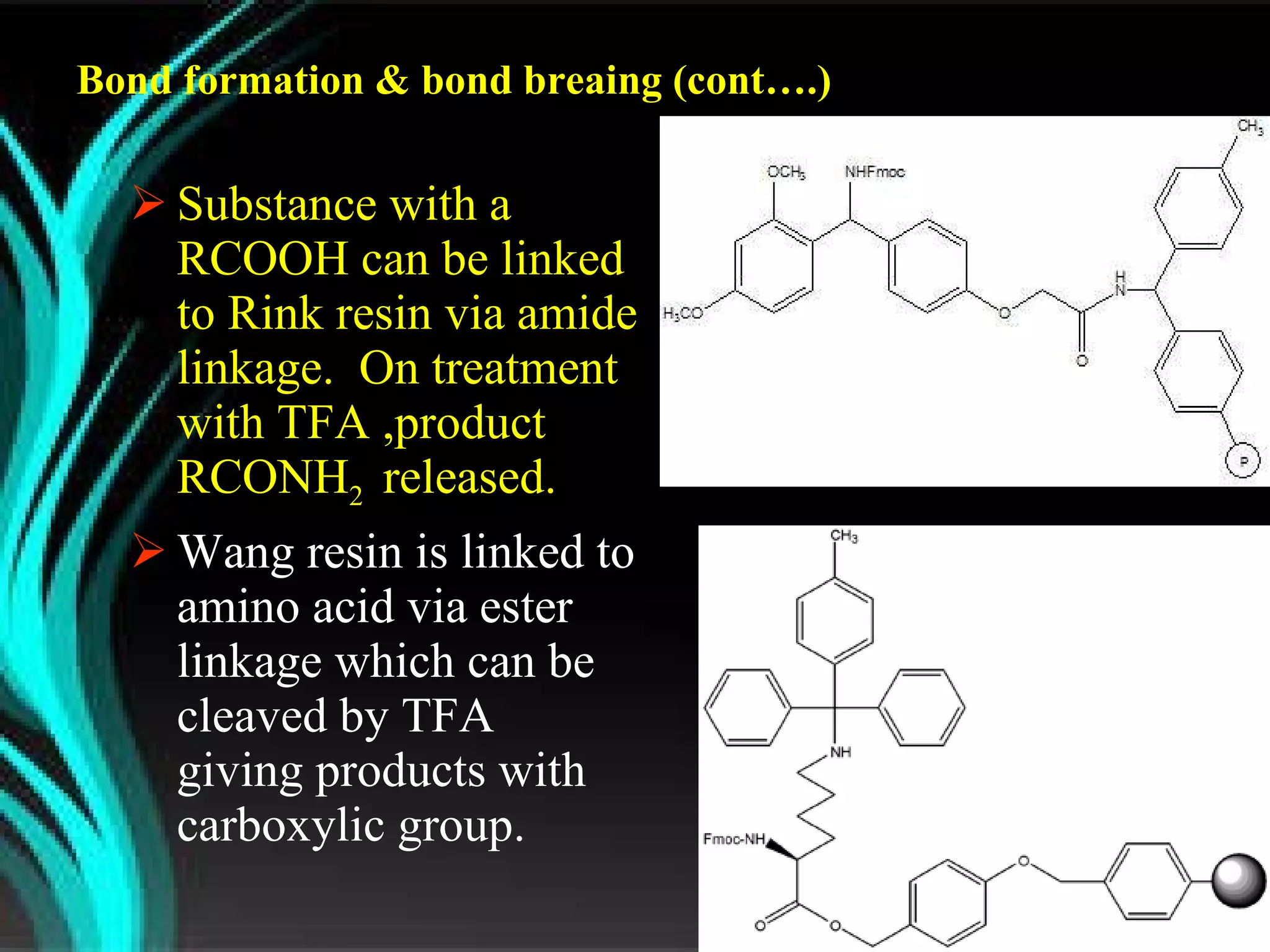
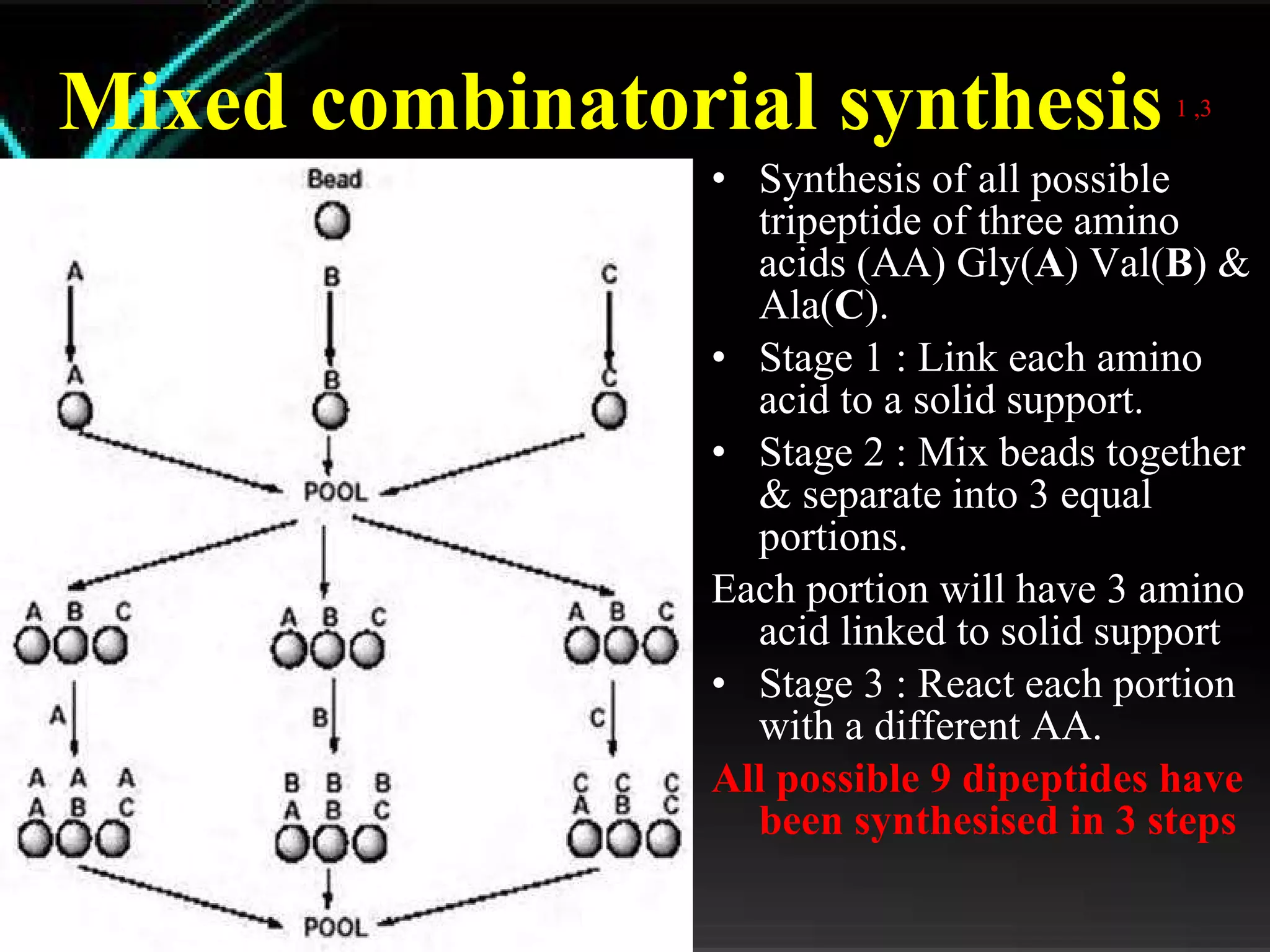
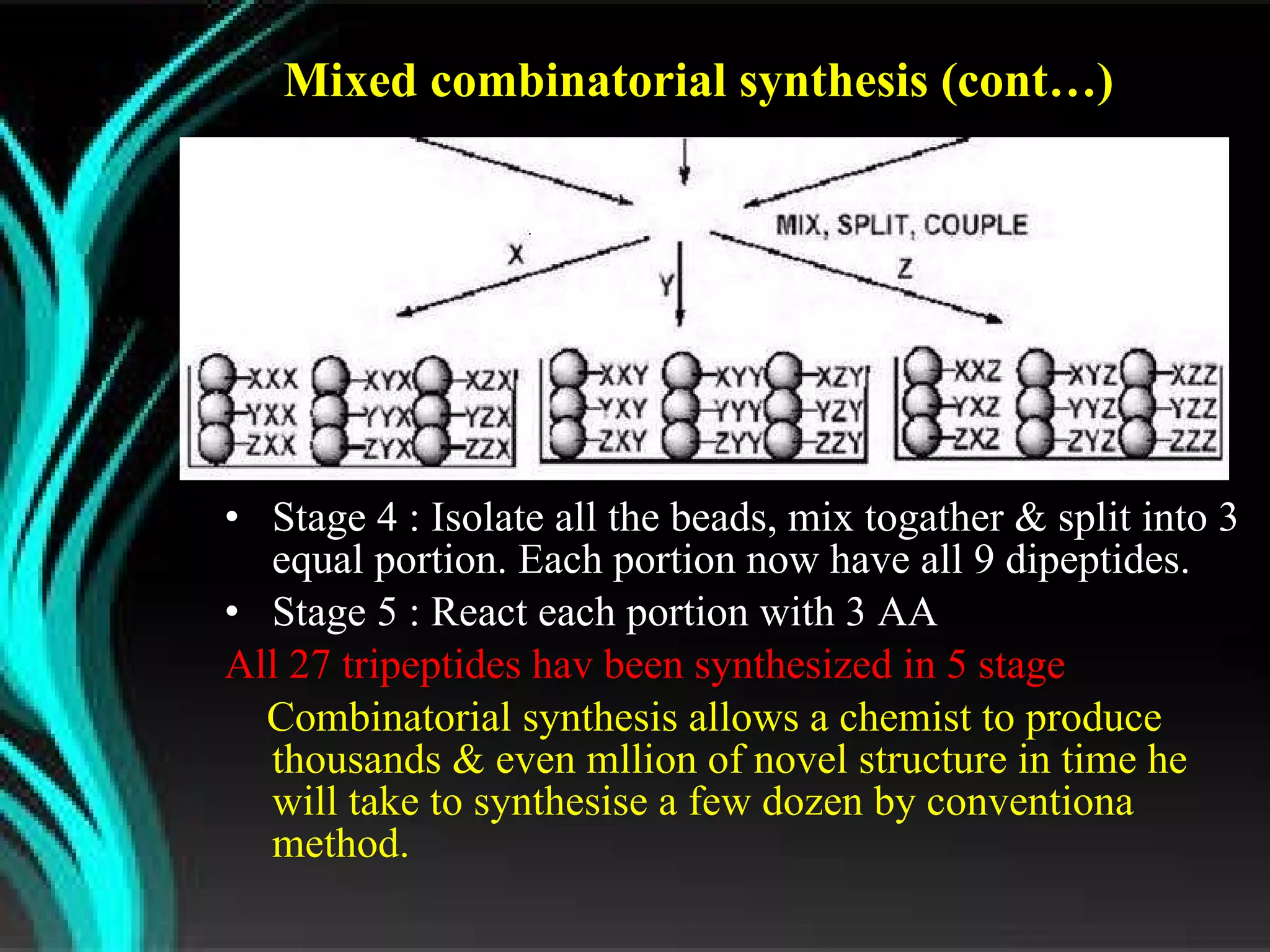
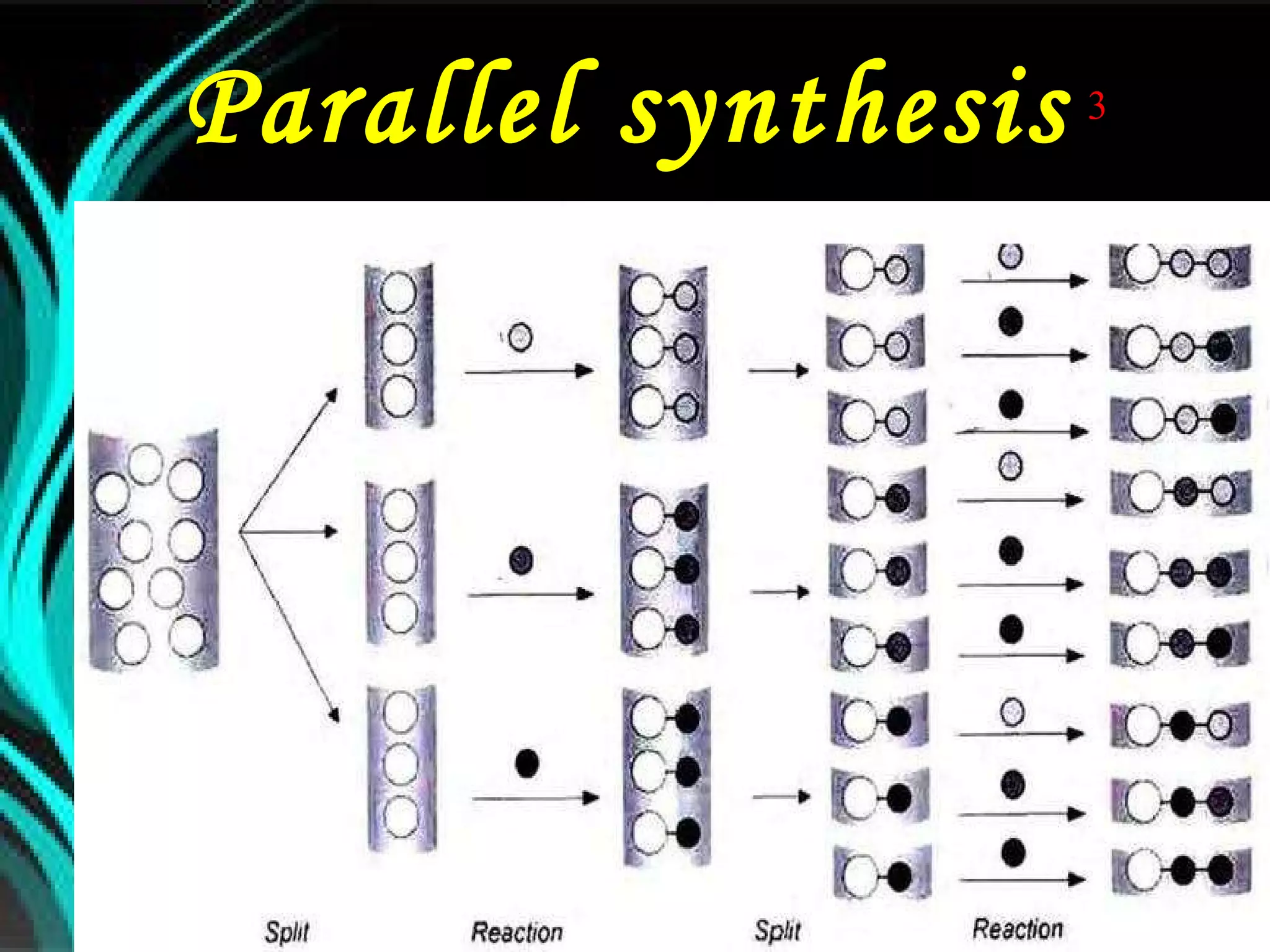
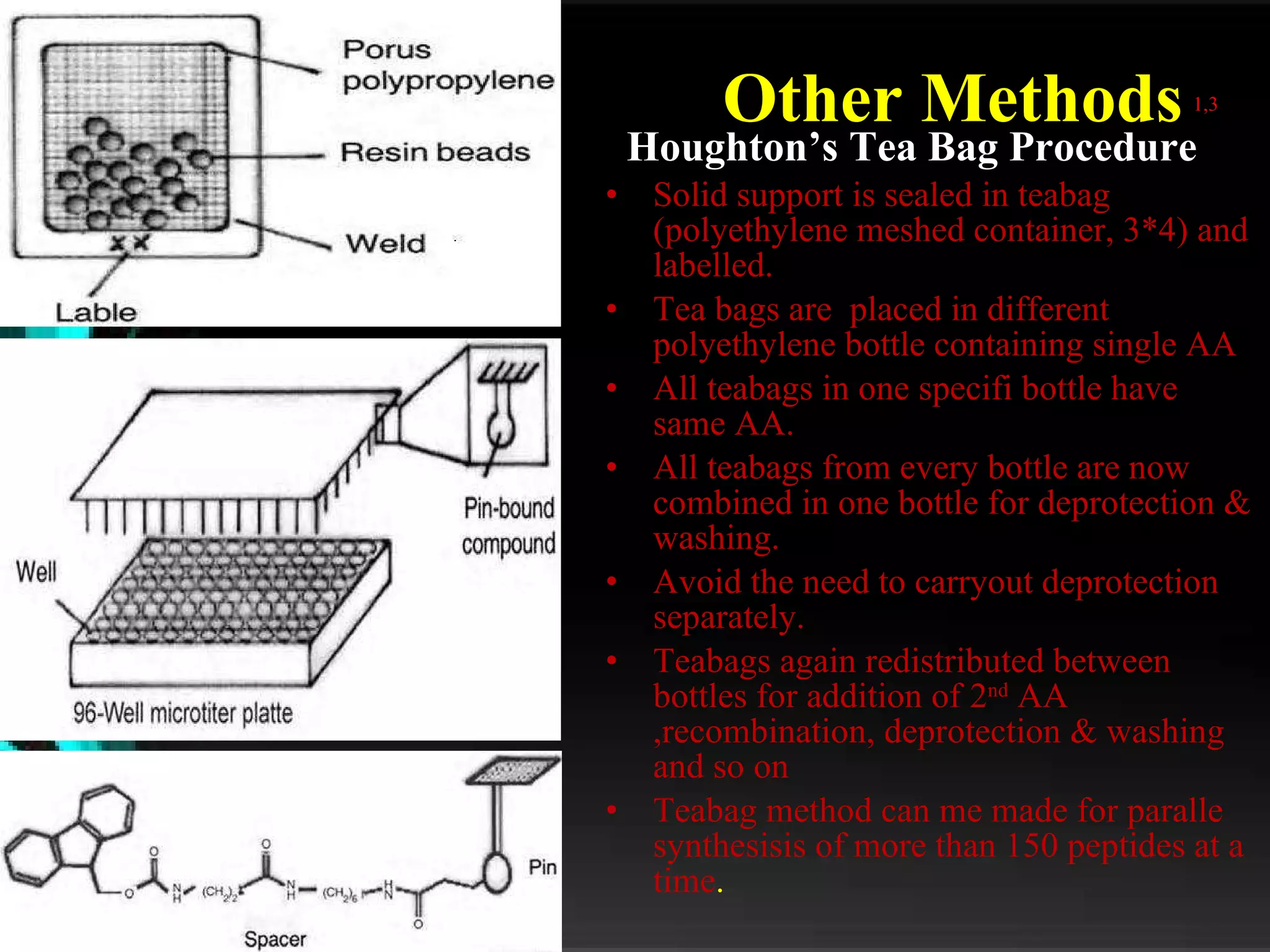
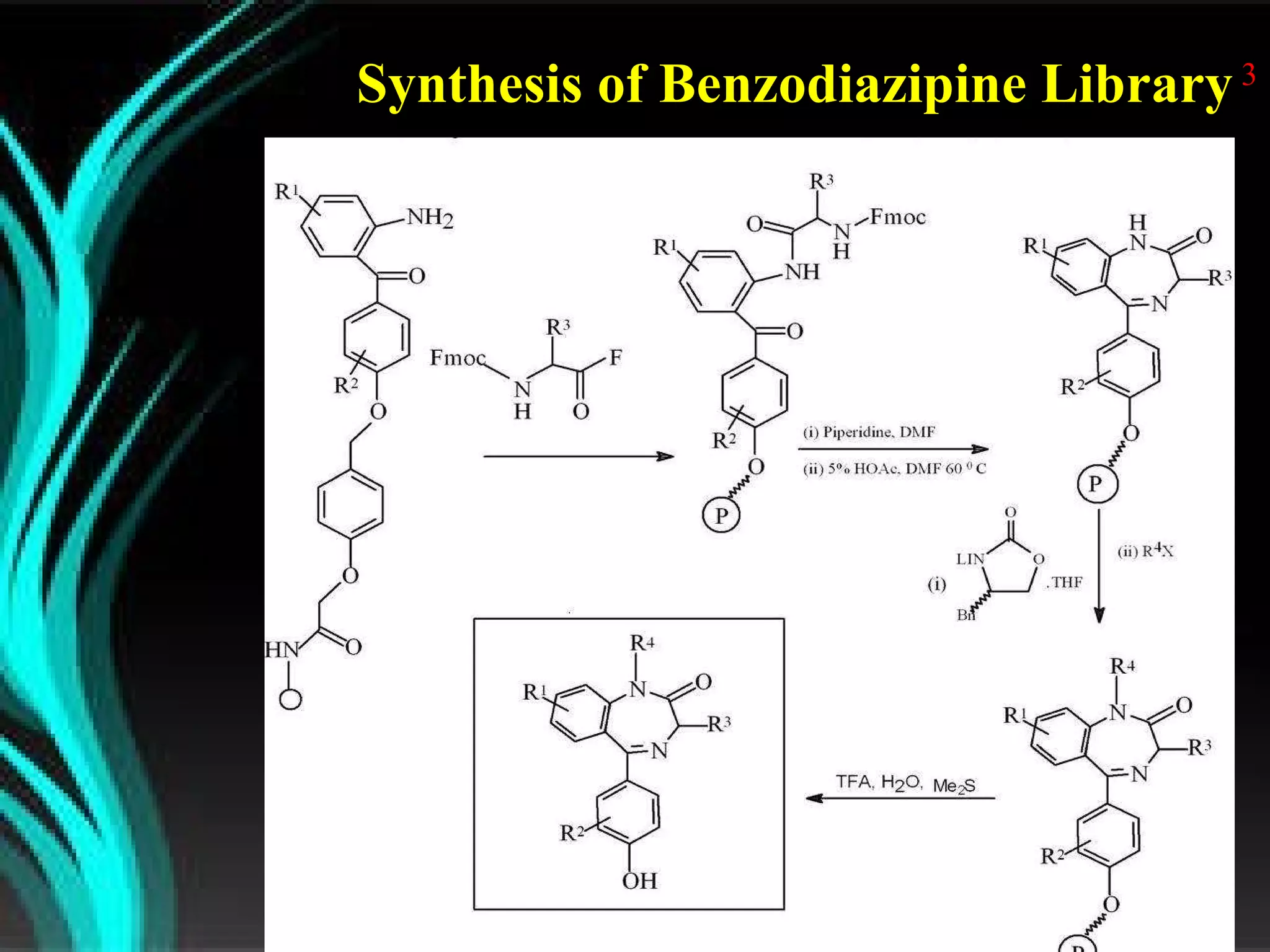
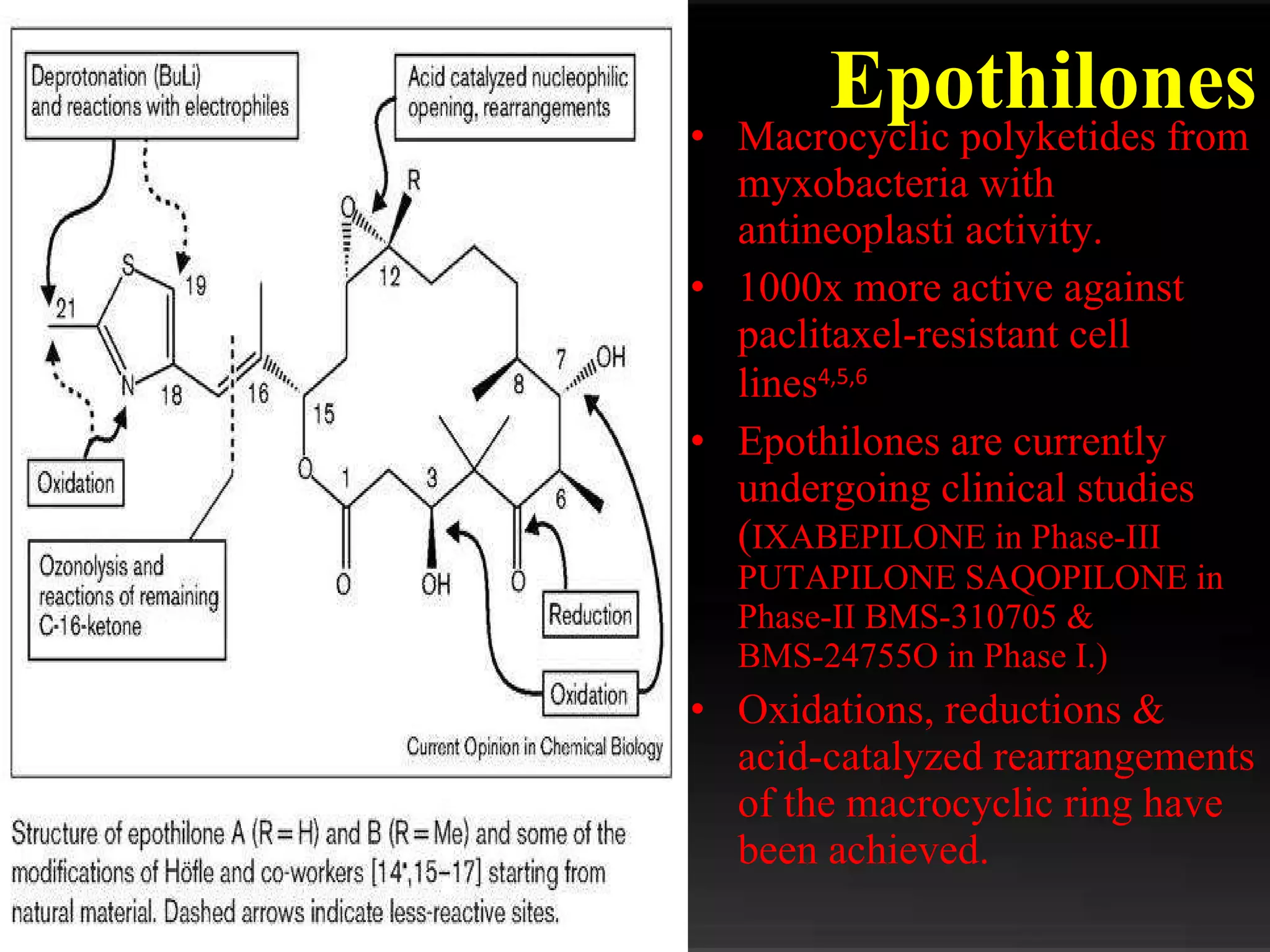
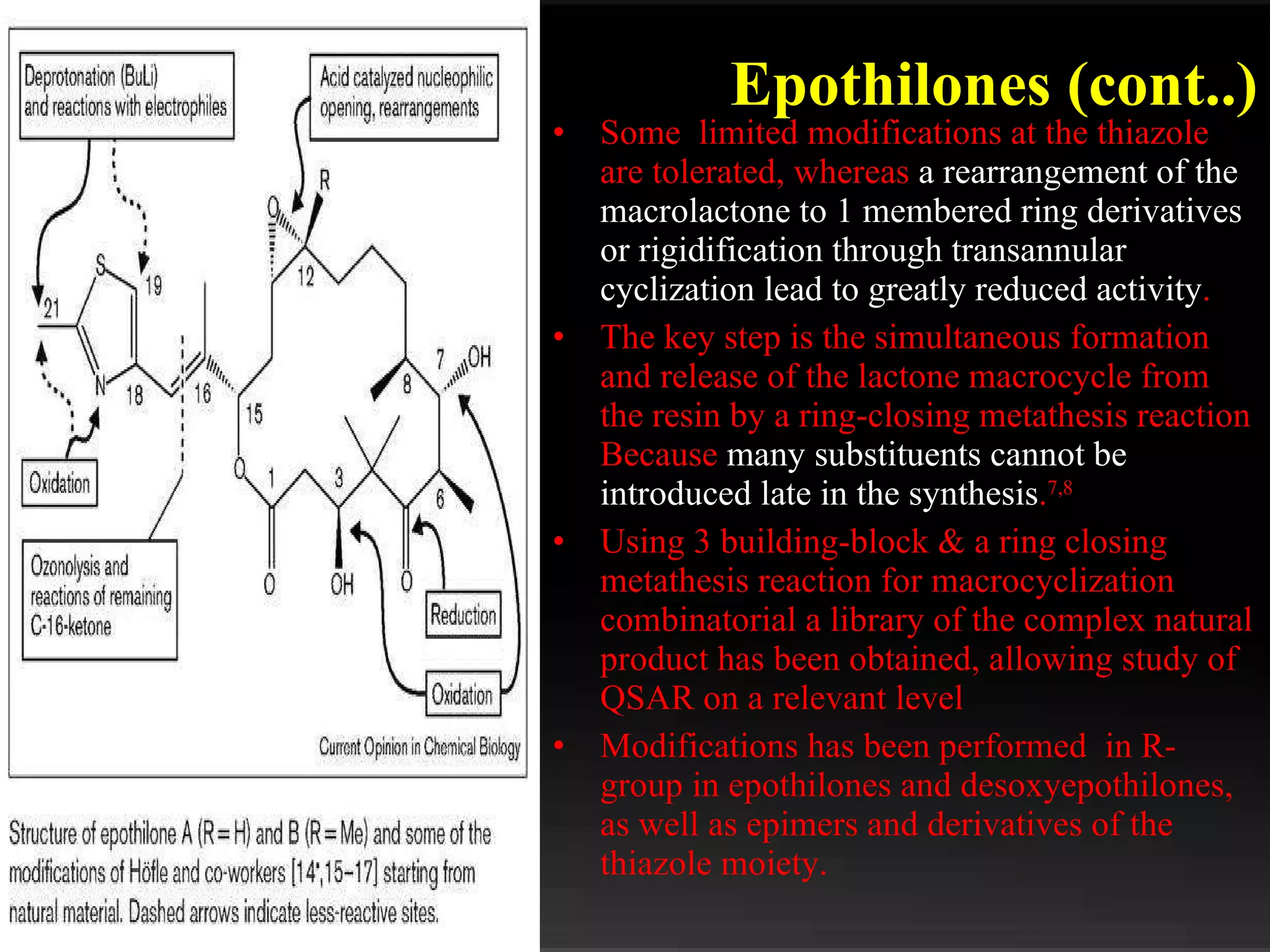
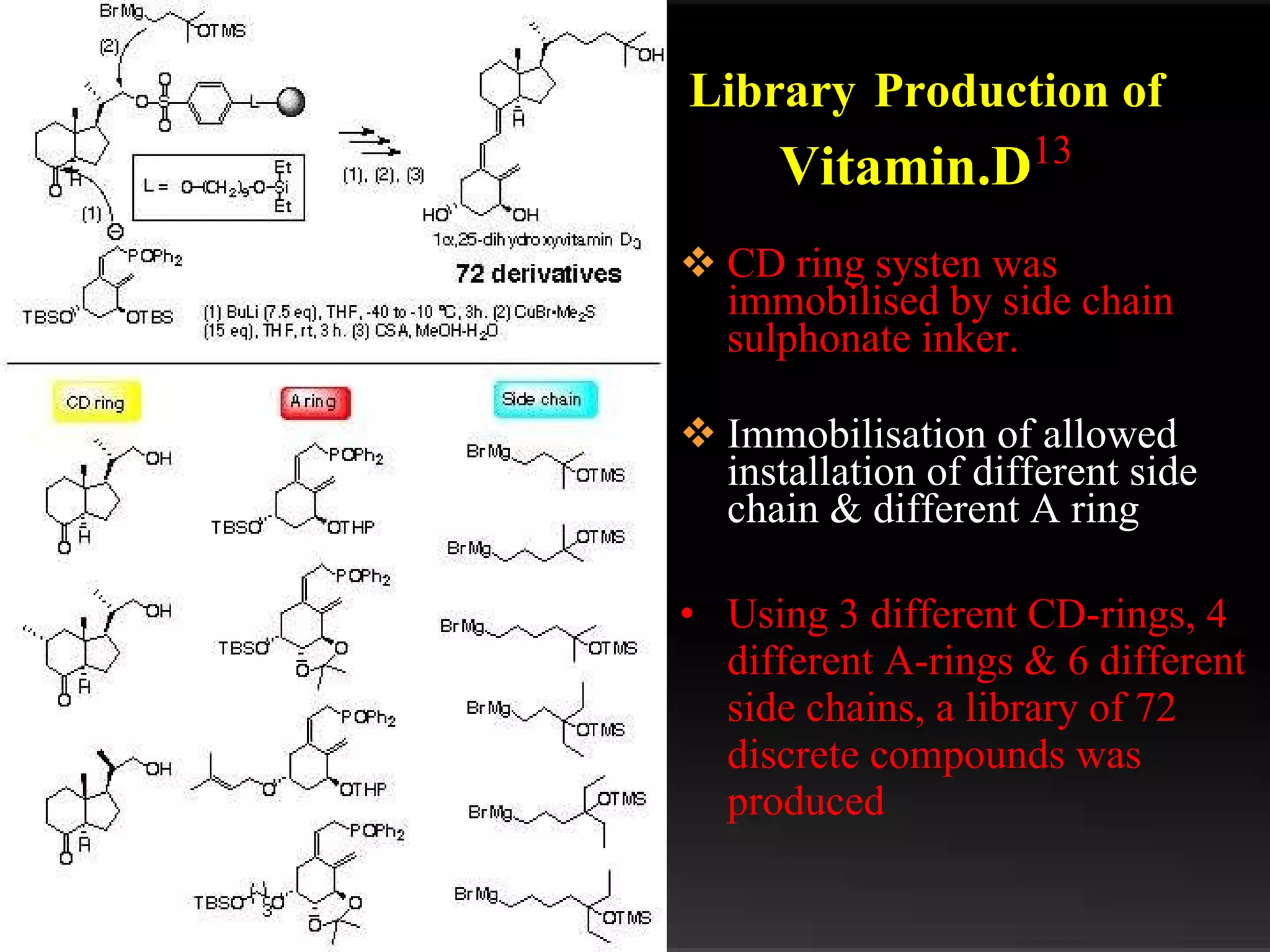
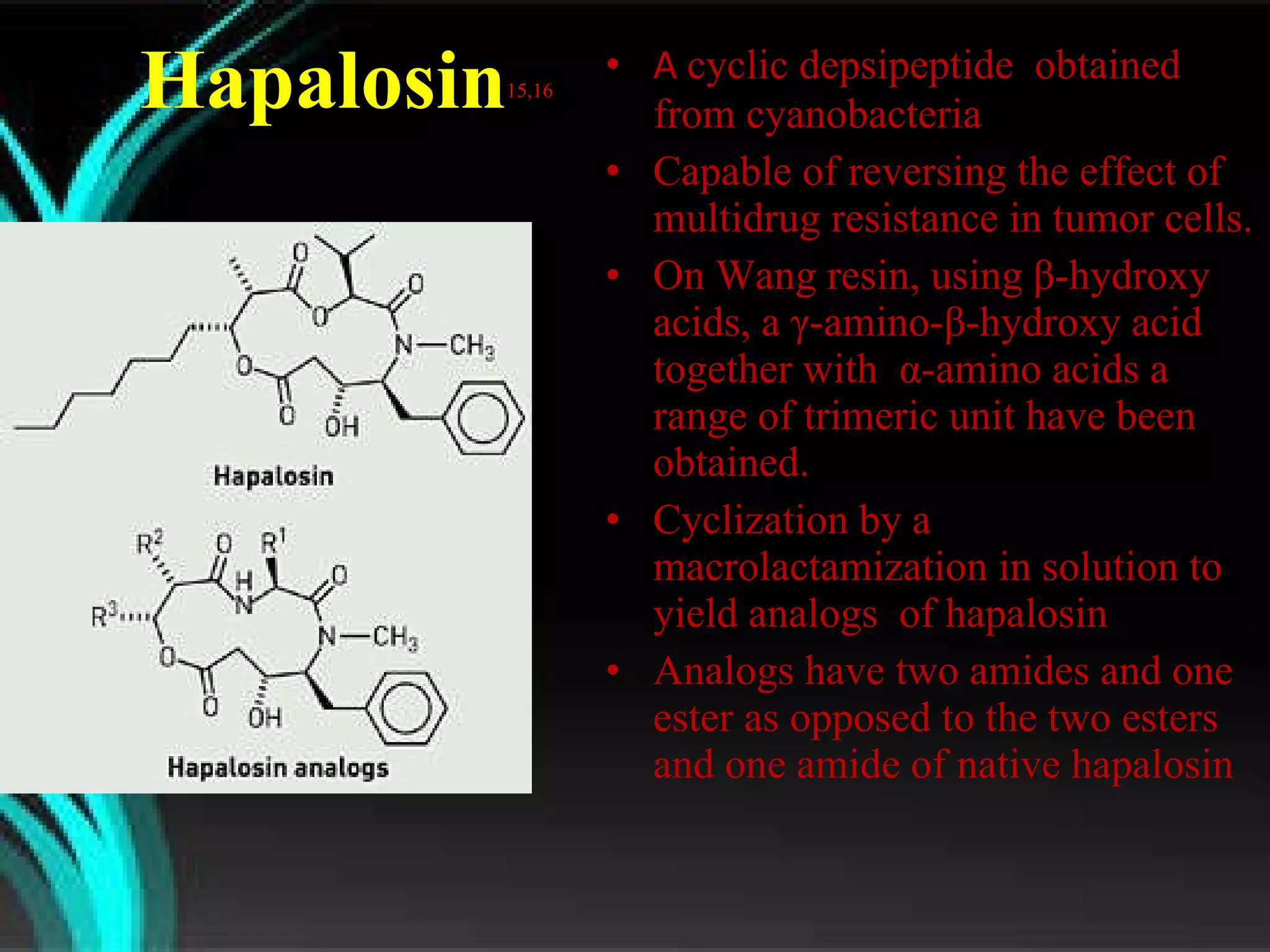
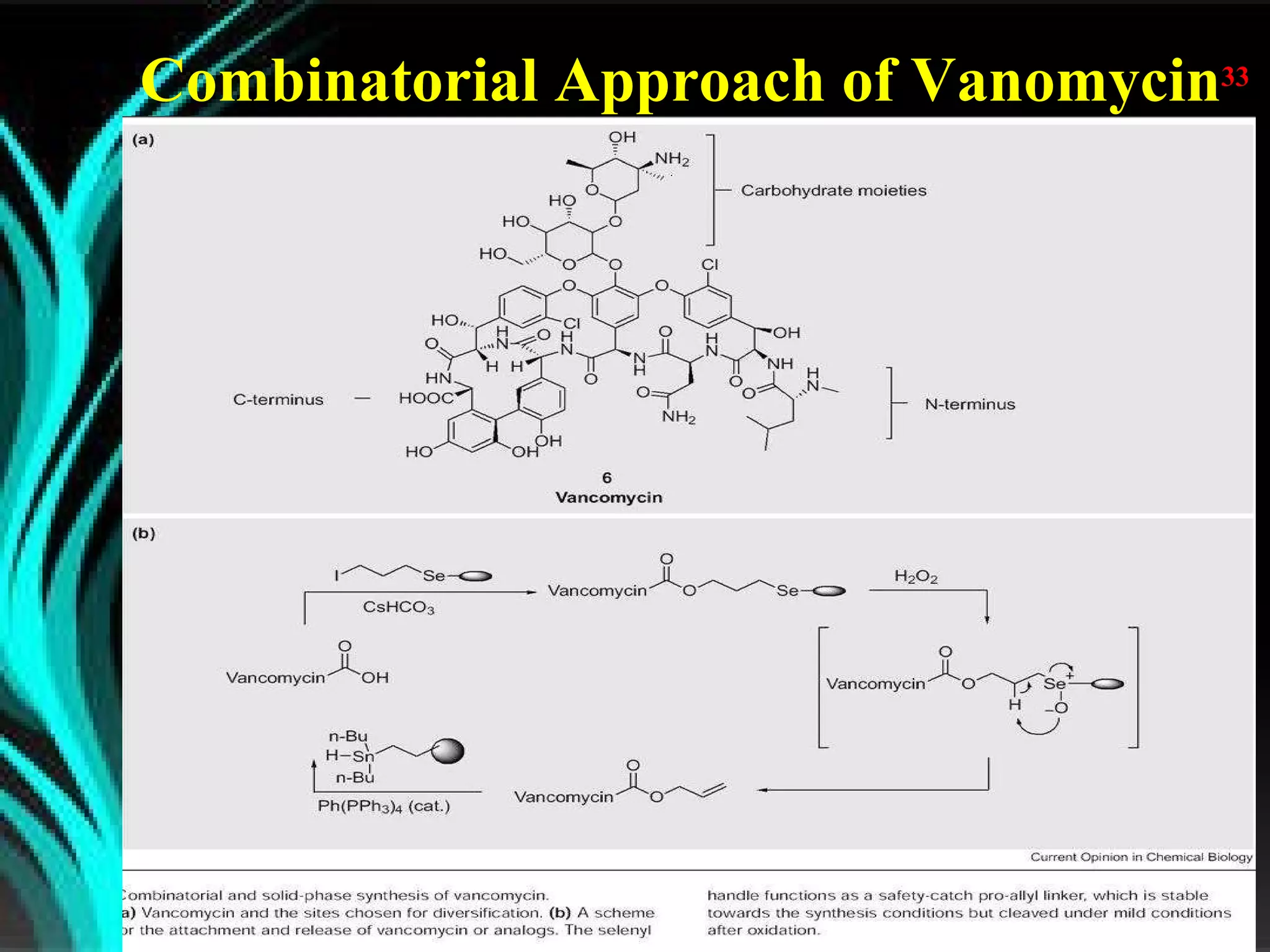
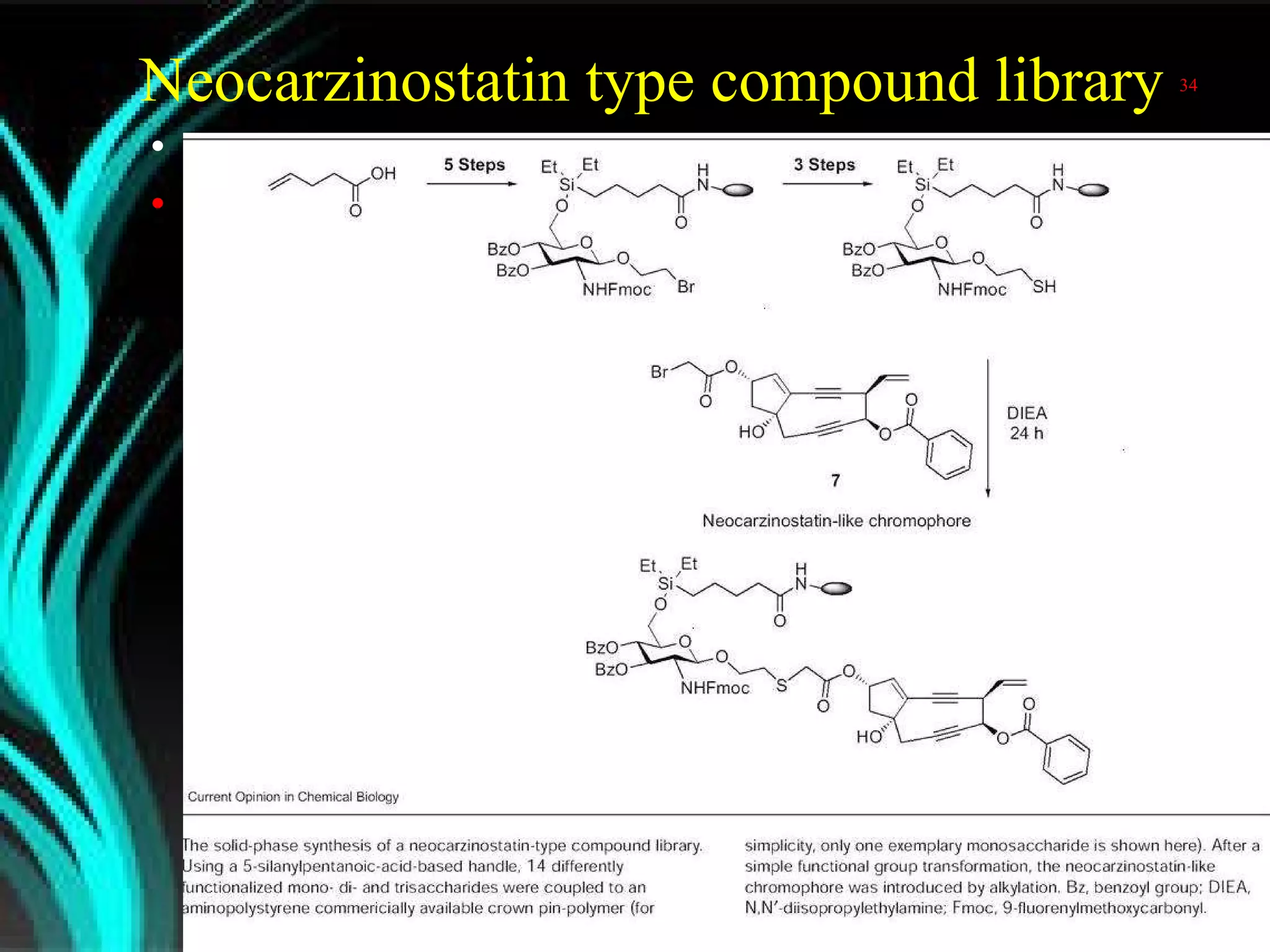
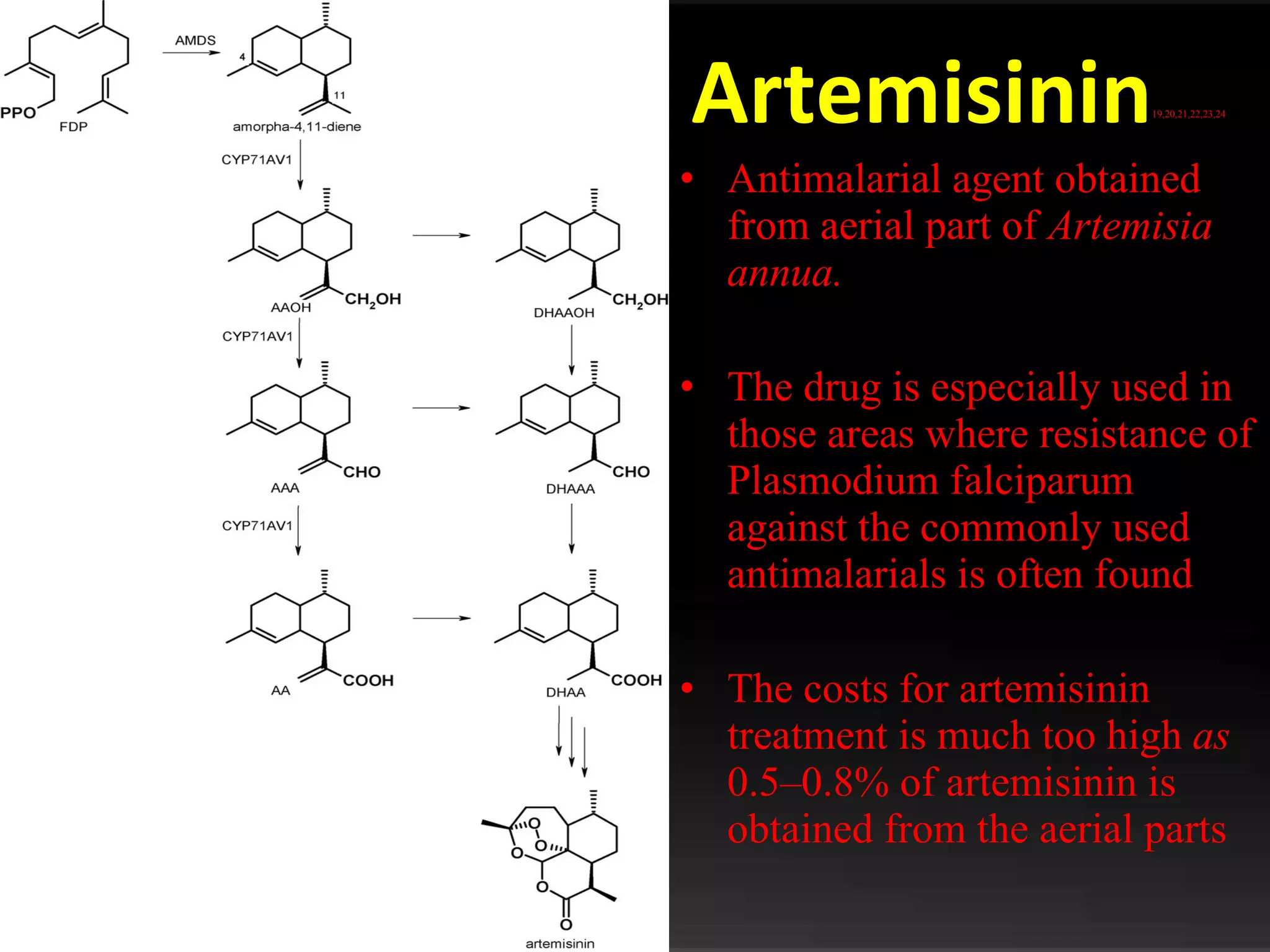
The document discusses natural product derived combinatorial libraries and their significance in drug discovery programs. It describes how combinatorial libraries are created through combinatorial synthesis techniques to generate large numbers of compounds that can be screened for biological activity. Libraries derived from natural products are particularly useful as natural products often have novel chemical structures not easily synthesized in labs. The document outlines methods for combinatorial synthesis, including solid phase and parallel synthesis techniques, and how libraries can be screened to identify potential drug candidates.