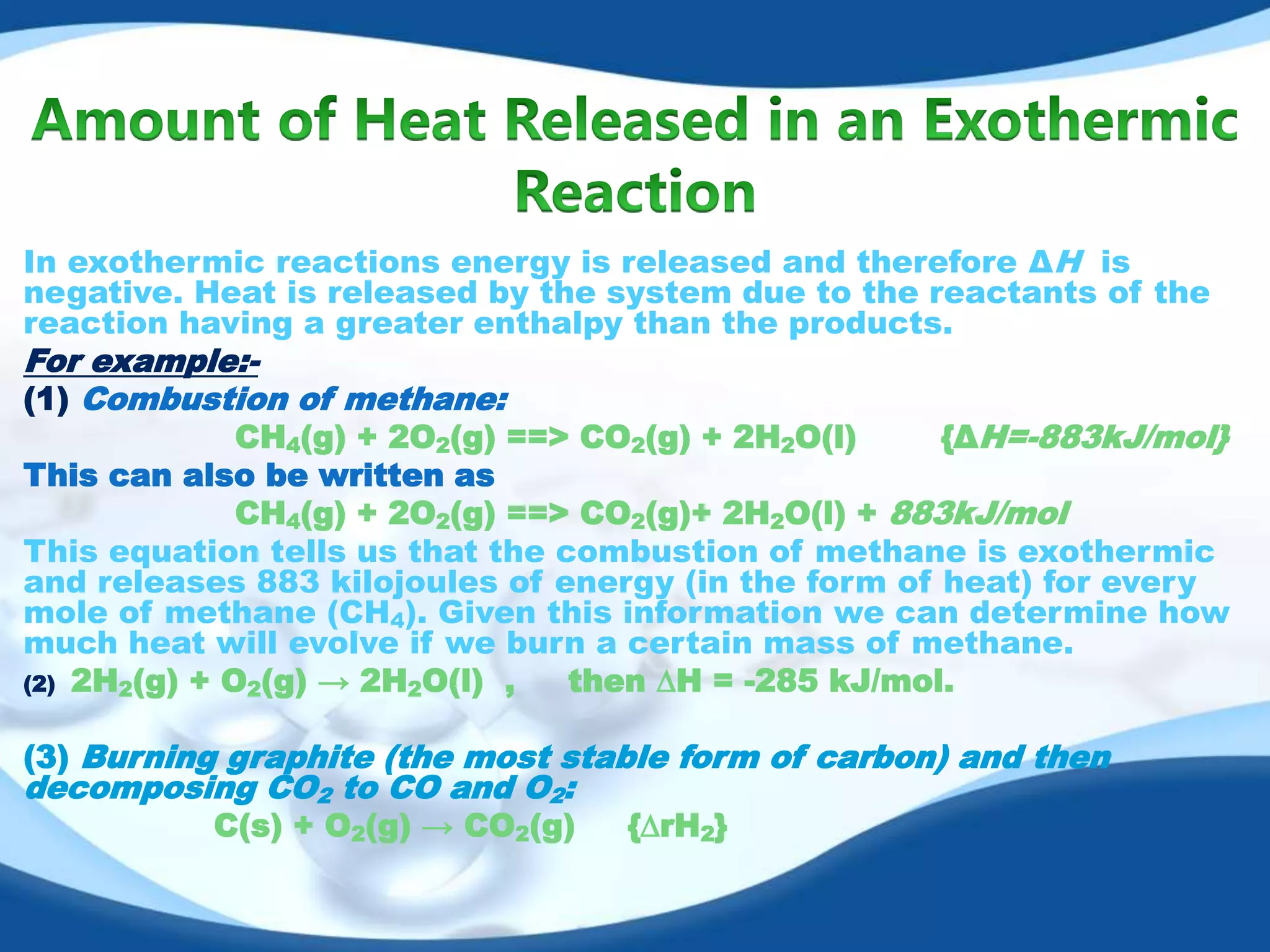
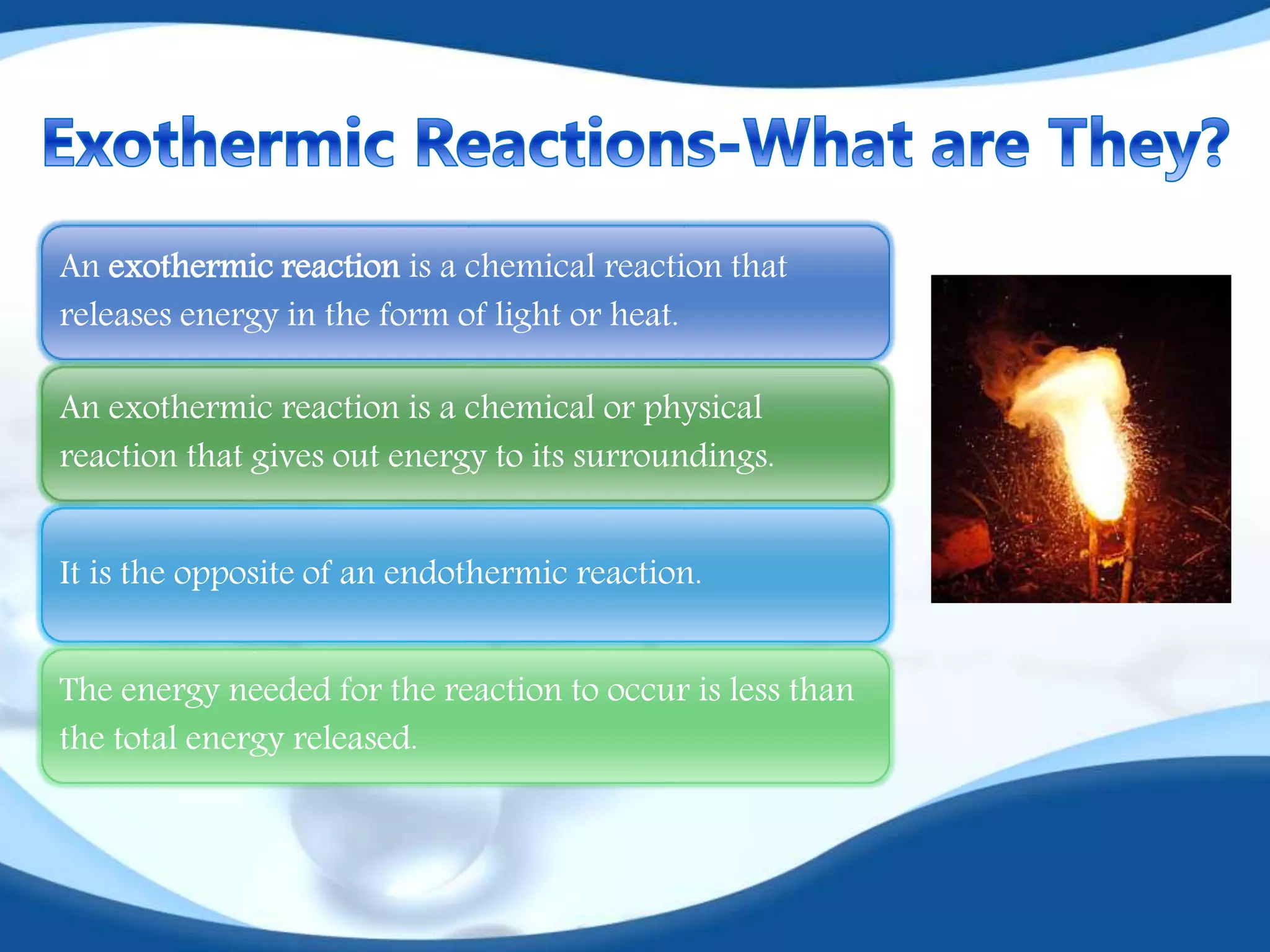
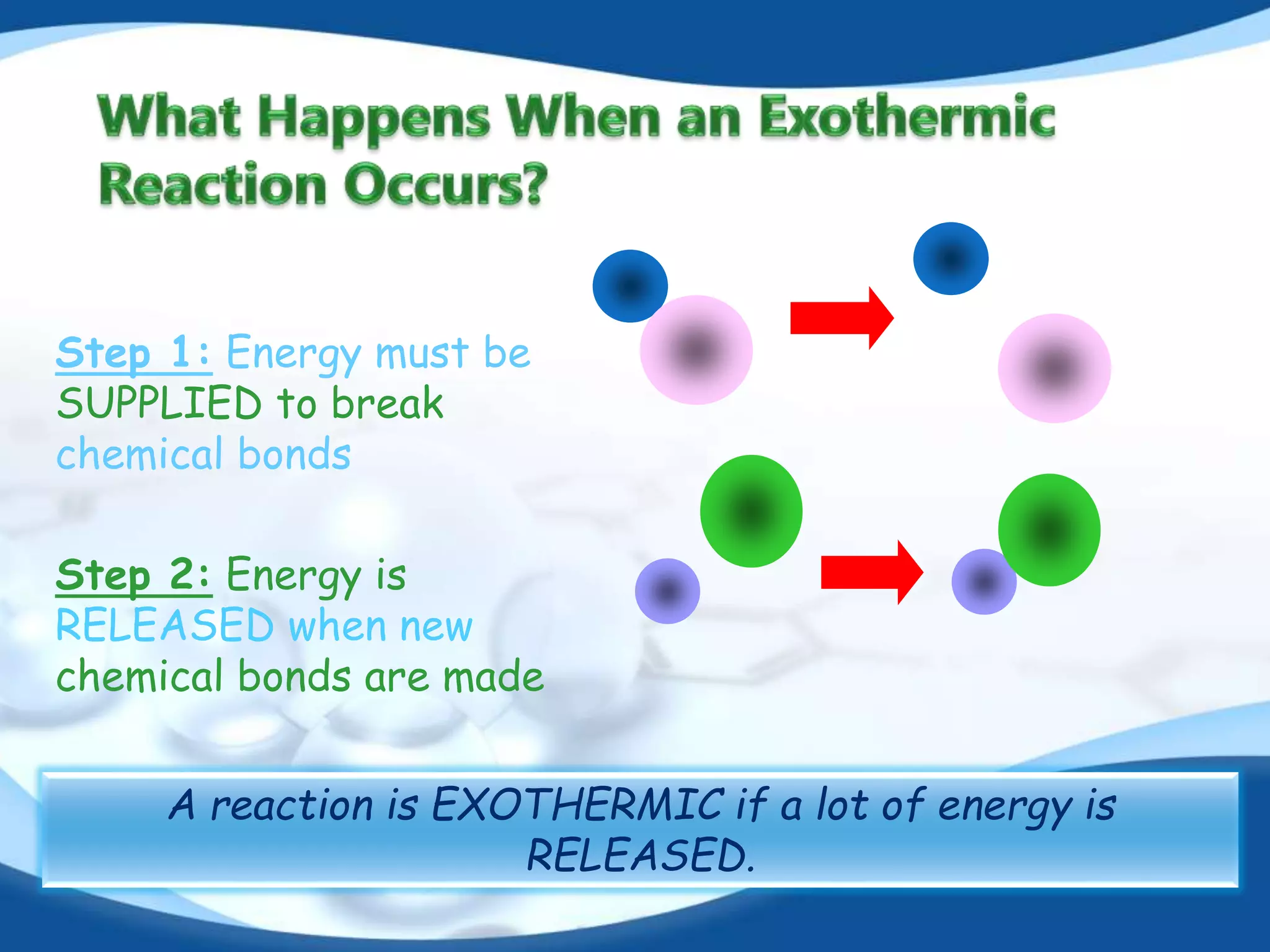
The document discusses exothermic reactions. It defines an exothermic reaction as a chemical reaction that releases energy in the form of heat or light. When an exothermic reaction occurs, energy is released to the surroundings. Examples of exothermic reactions given include respiration, burning of fuels like coal and natural gas, formation of water and ammonia, and the formation of slaked lime. Daily life examples provided are combustion, photosynthesis, and the formation of table salt. The document also discusses how the amount of heat released can be calculated using enthalpy change values and explains that exothermic reactions have a negative enthalpy change.


![• C(s)+ O2(g) →CO2(g) [Burning of Coal]
• 3H2 + N2 →2NH3 + Heat [Formation of Ammonia]
• 2H2(g) + O2(g)→H2O(l) [Formation of Water]
• 2SO2 + O2→ 2SO3 + Heat [Formation of Sulphur Trioxide]
• CH4(g) + 2O2(g)→CO2(g)+2H2O(g) [Burning of Natural Gas]
• CaO(s) + H2O(l)→Ca(OH)2(aq) [Formation of Slaked Lime]
• C6H12O6(aq) + 6O2(aq) → 6CO2(aq) + 6H2O(l) + energy [Respiration]](https://image.slidesharecdn.com/exothermicreactions-140728100930-phpapp01/75/Exothermic-reactions-6-2048.jpg)